Method for producing glucuronic acid and/or glucuronolactone
A technology of glucuronolactone and glucuronic acid, which is applied in the field of preparation of glucuronic acid and/or glucuronolactone, can solve the problems of high cost, high price, and low hydrolysis ability, and achieve Excellent production efficiency and low equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The present invention is further described in detail through the following examples, but the present invention is not limited thereto.
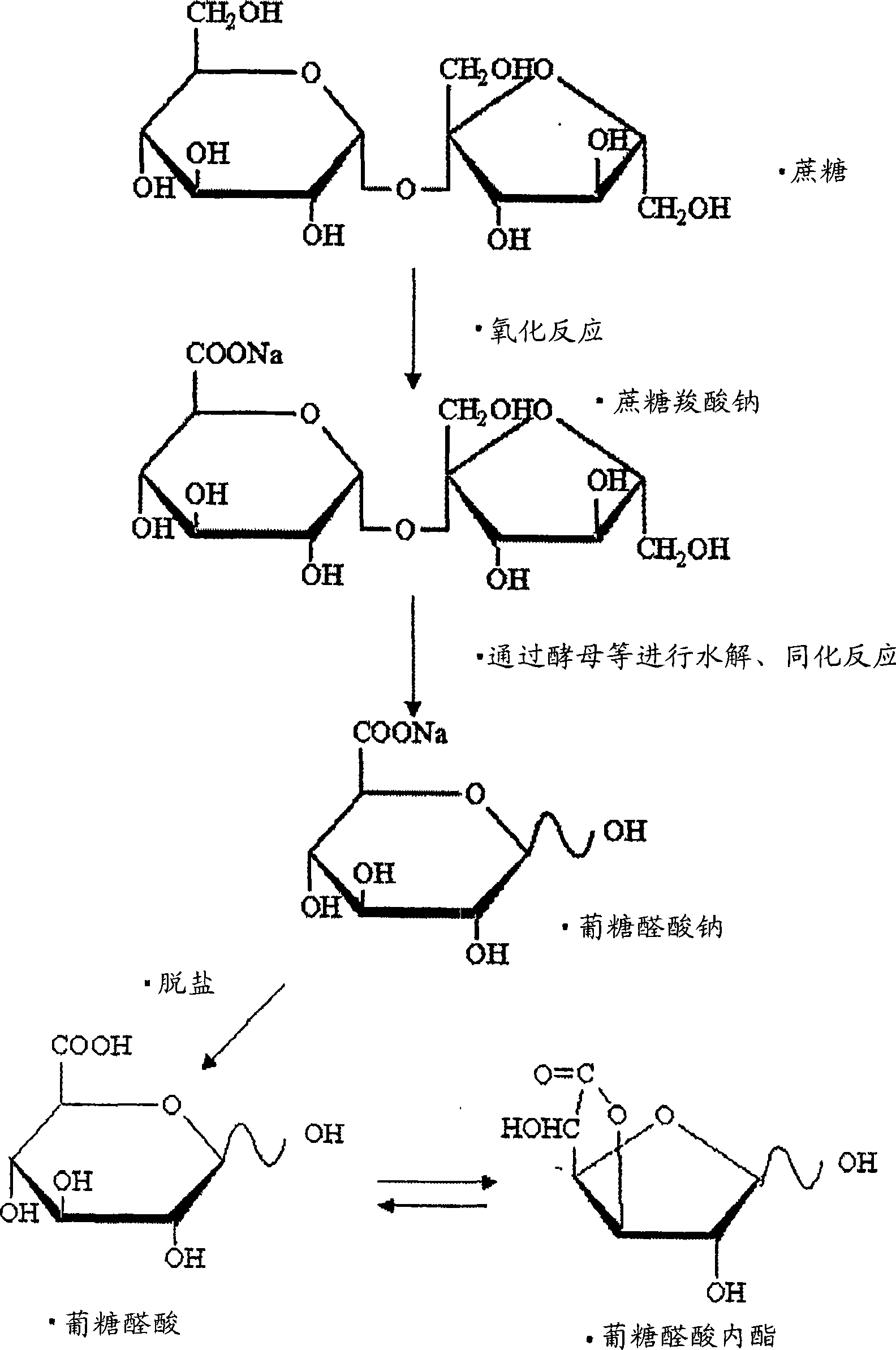
[0067] (1) Preparation of sucrose carboxylic acid sodium salt
[0068] According to the method described in Example 1 of Japanese Patent No. 3556690, an oxidase-producing bacterium was used to prepare a solution of an equivalent amount of sucrose carboxylate sodium salt (glucurono-fructoside) from 360 kg of sucrose (white sugar). That is, add 30kg sucrose and 250L water to the fermenter, dissolve, then add 50 L of Pseudogluconobacter saccharoketogenes washing bacteria liquid prepared in the same amount of fermenter to the solution prepared above, so that the total amount is 300L. Next, at 32° C., while stirring at 200 rpm, air was blown at a rate of 100 L / min, and the reaction was carried out for 24 hours to obtain the target sodium sucrose carboxylic acid salt. Then remove the bacterium, use again according to whether there is activi...
Embodiment 2
[0079] According to the method for Example 1, an equivalent amount of potassium salt of sucrose carboxylate was prepared from 15 kg of sucrose.
[0080] 100 g of potassium salt of sucrose carboxylate was taken therefrom, 7 g of commercially available baker's yeast (dry yeast) was added thereto, and hydrolysis reaction and assimilation reaction were performed at 37°C.
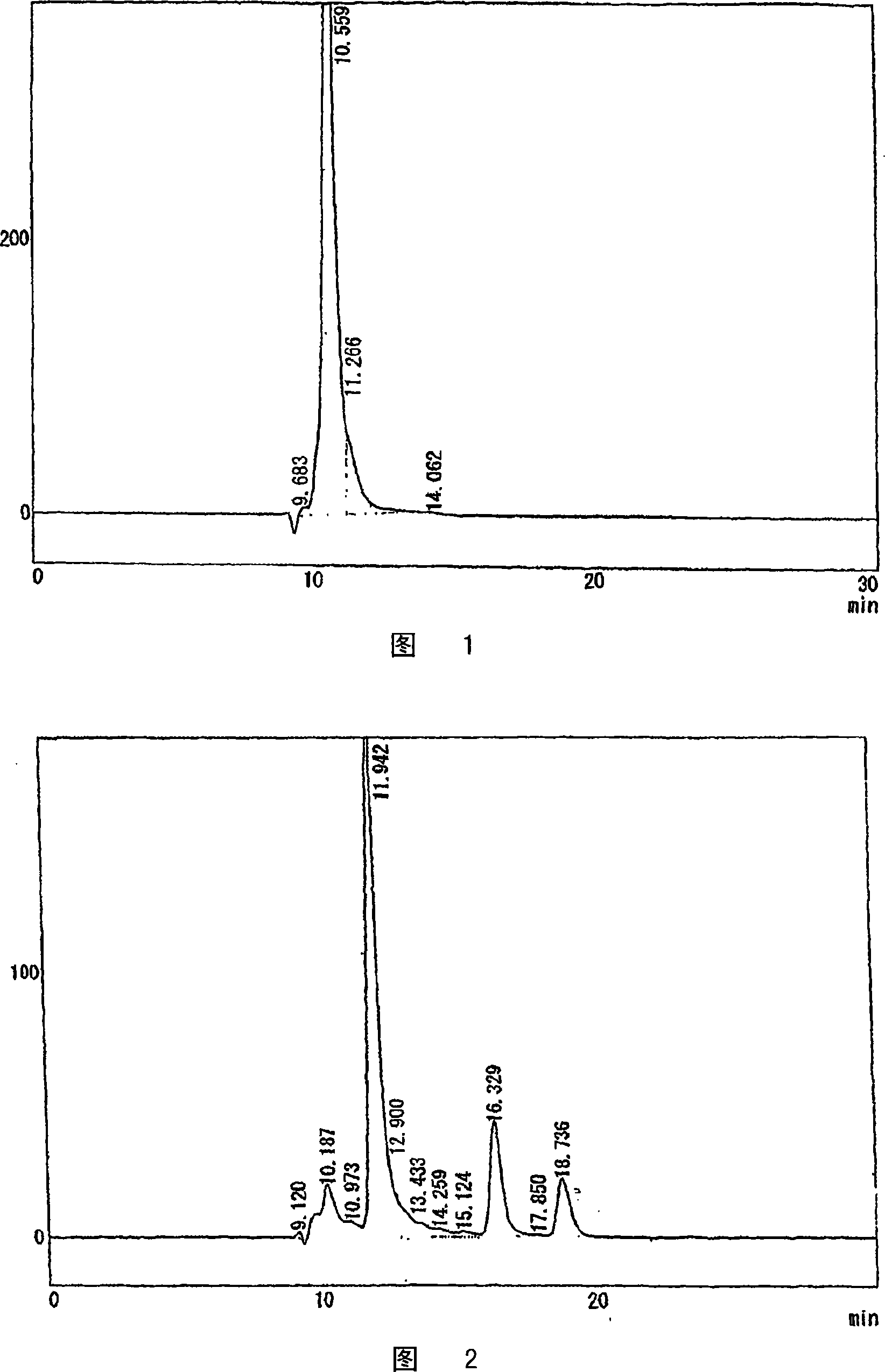
[0081] As a result, sucrose carboxylic acid was hydrolyzed within 24 hours to obtain a solution containing glucuronic acid and glucuronolactone. Then, the solution was passed through 1L activated carbon column (made by Egret, Japan EnviroChemicals, Ltd.), 1L basic ion exchange resin (Amberlite IRA-96SB, manufactured by Organo Corporation), 3L strongly acidic ion exchange resin (Diaion PK-216, manufactured by Mitsubishi Chemical Co., Ltd.), decolorized and desalted.
[0082] The desalted solution was concentrated to 65% at 40° C., 0.2 g of glucuronic acid seed crystal was added, and cooled naturally. The precip...
Embodiment 3
[0085] According to the method described in Example 1, an equivalent amount of sucrose carboxylic acid sodium salt was prepared from 15kg raw sugar.
[0086] Then also according to the method of Example 1, 15kg of sucrose carboxylic acid sodium salt solution was concentrated to 30%, and then 3% baker's yeast (Oriental Yeast Co., Ltd. preparation, FD-1) was added in terms of solid content, at 37°C The reaction was carried out for 72 hours.
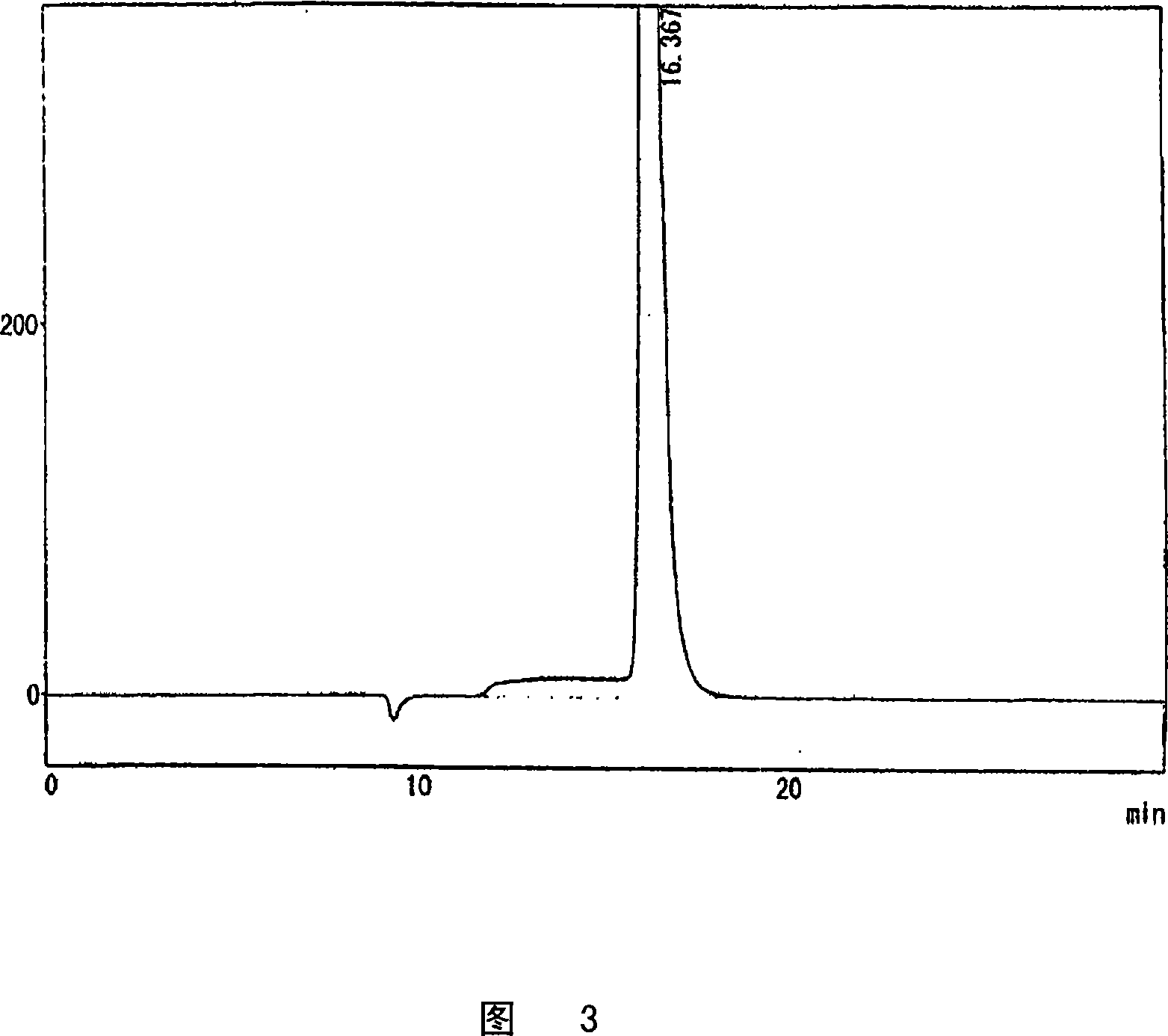
[0087] The reaction solution is also passed through an activated carbon column and a desalting resin column for decolorization and desalination. The resulting desalted solution was concentrated to 58% at 50° C., and 20 g of glucuronolactone seed crystals were added to precipitate crystals. Then, centrifugation was performed to obtain 2 kg of glucuronolactone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com