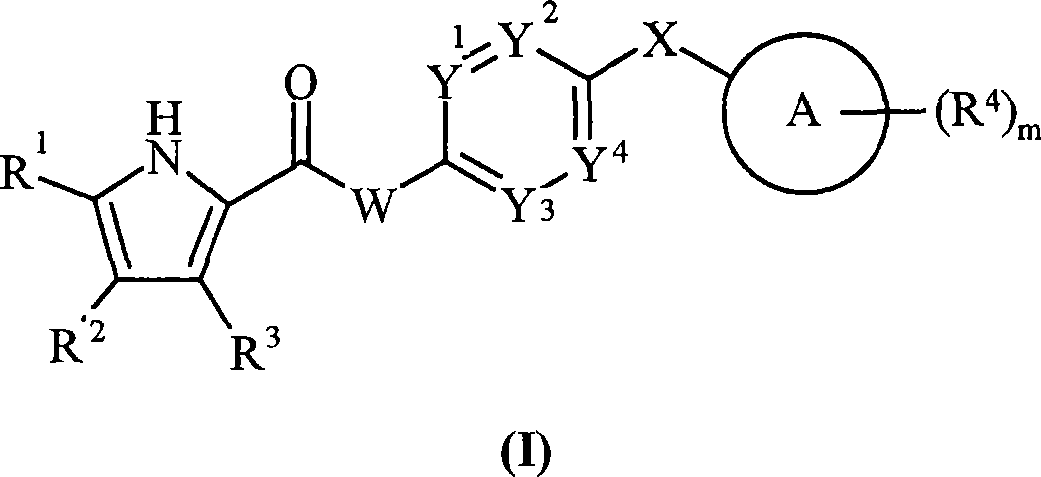
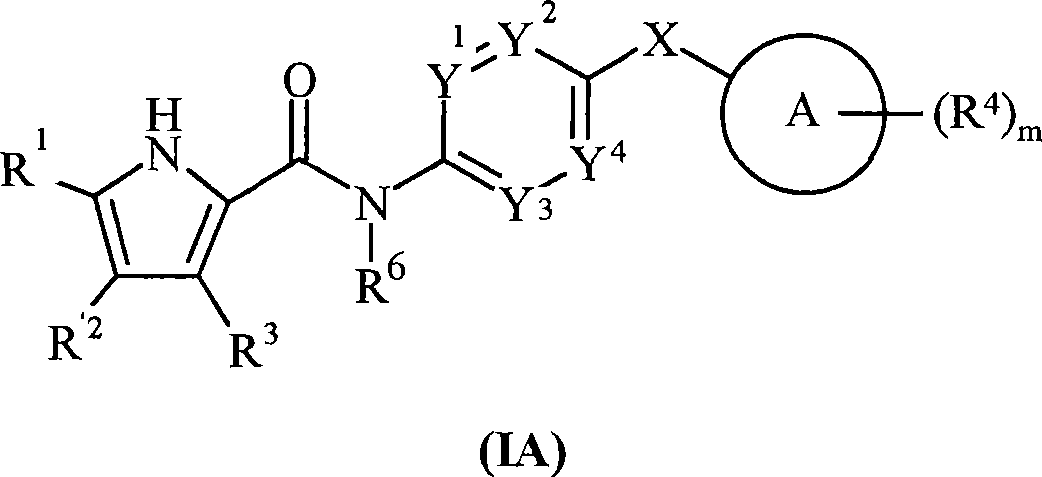
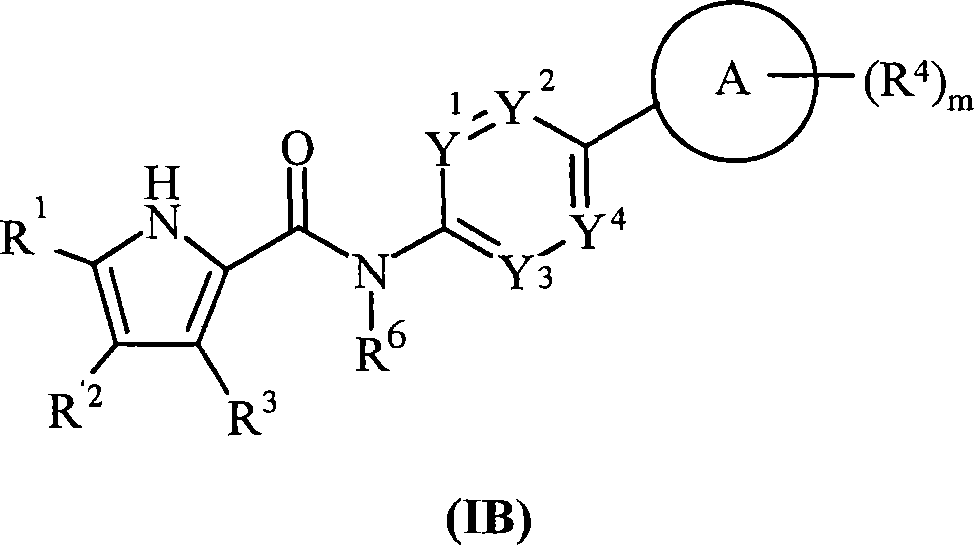
Pyrrole derivatives as DNA gyrase and topoisomerase inhibitors
An alkyl, selected technology, applied in the field of pyrrole derivatives as DNA gyrase and topoisomerase inhibitors, can solve the problem of poor antibacterial drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 30
[0396] The MIC of the compound of Example 30 against Streptococcus pneumoniae was 1 μg / ml. Other examples are listed in the table below.
[0397] Example number
MIC SPN548
MIC SAU516
MIC HIN446
23
25
26
27
36
0.13
1
1
0.13
0.25
4
2
32
2
2
2
4
8
2
1
[0398] The present inventors have found that the compounds of the present invention inhibit bacterial DNA gyrase, so that they have antibacterial effects of interest.
[0399] According to another feature of the invention, there is provided a method of producing an antibacterial effect in a warm-blooded animal in need of such treatment, such as a human, the method comprising administering to said animal an effective amount of a compound of the invention or a pharmaceutically acceptable salt thereof.
[0400] According to another feature of the present invent...
Embodiment 1
[0460] [4-(4-{{(3,4-dichloro-5-methyl-1H-pyrrol-2-yl)carbonyl]amino}phenyl)-1H-1,2,3-tri Azol-1-yl] ethyl acetate
[0461] Make 3,4-dichloro-N-(4-ethynylphenyl)-5-methyl-1H-pyrrole-2-carboxamide (intermediate 11; 1g, 3.4mmol), CuI (1.3g, 6.8mmol , 2 eq) and ethyl azidoacetate (880 mg, 6.8 mmol, 2 eq) were mixed and dissolved in DIEA (1.3 g, 10.1 mmol, 3 eq) and 1,4-dioxane (20 ml). The resulting slurry was heated to 100 °C for 6 h. The reaction was monitored by LC / MS. The solid was filtered and the mother liquor was concentrated to a foam. MS(ES)(M+H) + =422,424; NMR: 1.22(t,3H), 2.24(s,3H), 4.18(q,2H), 5.54(s,2H), 7.74(dd,4H), 8.51(s,1H), 9.54 (s, 1H), 12.2 (s, 1H).
Embodiment 2
[0463] [4-(4-{[(3,4-Dichloro-5-methyl-1H-pyrrol-2-yl)carbonyl]amino}phenyl)-1H-1,2,3-tri Azol-1-yl]acetic acid
[0464] [4-(4-{[(3,4-dichloro-5-methyl-1H-pyrrol-2-yl)carbonyl]amino}phenyl)-1H-1,2,3-triazole-1 -yl]ethyl acetate (Example 1; 1.4 g, 3.4 mmol) was dissolved in a 1:1 mixture of EtOH (10 mL) and 2N KOH (10 mL). The reaction mixture was stirred at room temperature for 3 days. The solution was cooled to 0°C and acidified to pH 7 with 2N HCl. The mixture was concentrated to dryness and purified by HPLC (15-95% acetonitrile / 0.1% TFA for 35 min). The product fractions were collected, the acetonitrile was removed by rotary evaporation, the water was frozen and lyophilized. After isolation, 60 mg of the title compound were obtained. M / z 393, 395; NMR: 2.17(s, 3H), 5.25(s, 2H), 7.67(dd, 4H), 8.42(s, 1H), 9.46(s, 1H), 12.1(s, 1H), 13.4 (broads, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com