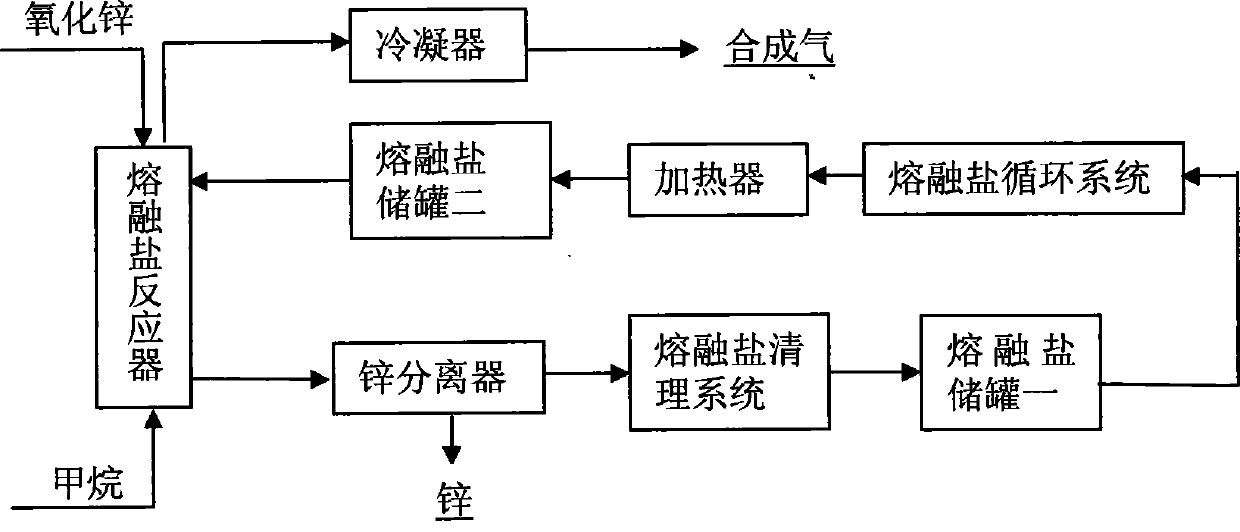
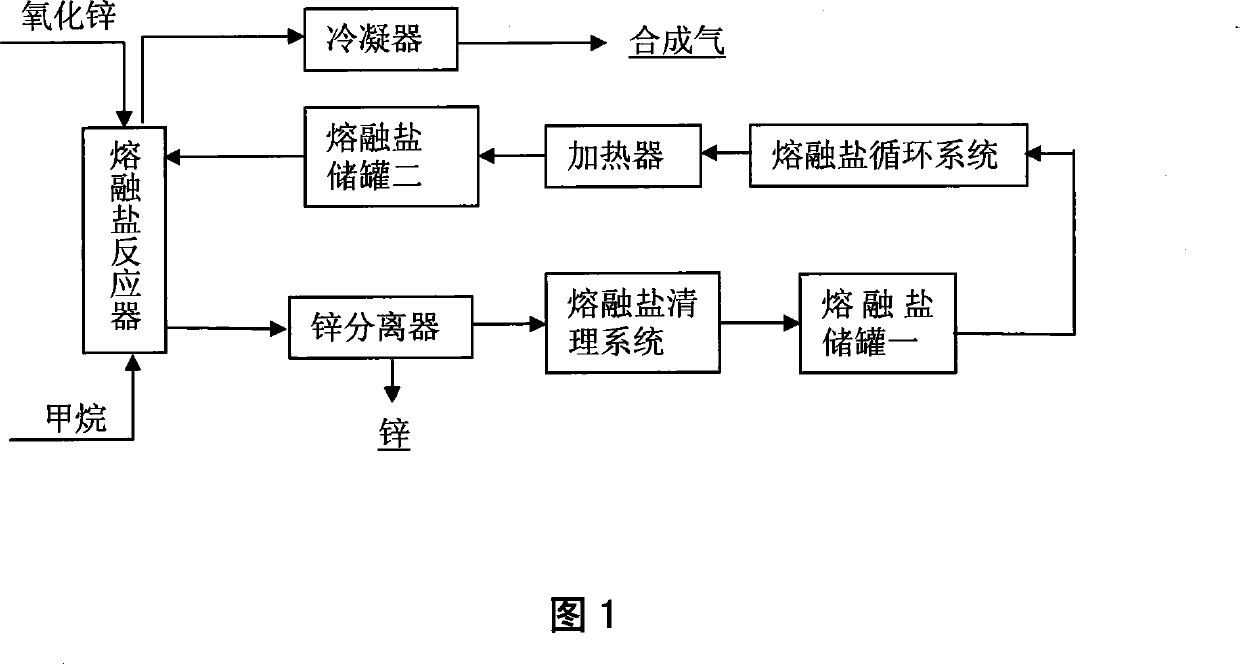
Method for preparing synchronously synthetic gas and metal zinc
A metal zinc and synthesis gas technology, applied in chemical instruments and methods, non-metallic elements, inorganic chemistry, etc., can solve the problems of low energy utilization efficiency, poor reaction efficiency, expensive investment and operation costs of oxygen production equipment, etc., and achieve improvement The heat utilization rate of the system, the uniformity of the reaction temperature field, and the effect of avoiding hot spots
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] ① Implementation conditions
[0021] Using zinc oxide as the oxygen carrier, sodium carbonate (Na 2 CO 3 ) and potassium carbonate (K 2 CO 3 ) as a molten salt system, industrial methane is the reaction raw material gas, and the reactor is a stainless steel reactor with a length of 550mm and an inner diameter of 28mm, and one end is sealed. The zinc oxide powder and the mixed carbonate are pre-ground evenly and put into the reactor, the mass of the zinc oxide powder accounts for 35% of the mass of the molten salt, the reactor is placed in a tubular electric furnace, and the temperature is raised to 1173K for heating for 2 hours; Put the air inlet and outlet pipes and the connected sealing caps in the reactor, the air inlet pipes go deep into the bottom of the reactor, tighten the sealing caps, the air outlet pipes first lead into a cold well, then into a container filled with water, check the airtightness, and then Nitrogen (N 2 ) for about 2 hours to empty the air...
Embodiment 2
[0025] ① Implementation conditions
[0026] Zinc oxide is used as the oxygen carrier, and the molten salt system selected is sodium carbonate (Na 2 CO 3 ), potassium carbonate (K 2 CO 3 ) and lithium carbonate (Li 2 CO 3 ), industrial methane is the reaction raw material gas, and the reaction is carried out in a stainless steel reactor with a length of 550mm and an internal diameter of 28mm, and one end is sealed. The zinc oxide powder and the mixed carbonate are pre-ground evenly and put into the reactor. The mass of zinc oxide powder accounts for 20% of the mass of the molten salt, the reaction temperature is 1223K, the temperature fluctuation range is ±1K, and the gas flow rate is 150Ncm 3 min -1 , the reaction time is 1 hour, and the reaction is an intermittent operation.
[0027] ②Implementation results
[0028] Through the composition analysis of the product gas, it is found that the gas composition is mainly hydrogen (H 2 ) and carbon monoxide (CO), the molar r...
Embodiment 3
[0030] ① Implementation conditions
[0031] Zinc oxide is used as the oxygen carrier, and the molten salt system selected is potassium carbonate (K 2 CO 3 ) and lithium carbonate (Li 2 CO 3 ), industrial methane is the reaction raw material gas, and the reaction is carried out in a stainless steel reactor with a length of 550mm and an internal diameter of 28mm, and one end is sealed. The zinc oxide powder and the mixed carbonate are pre-ground evenly and put into the reactor. The mass of zinc oxide powder accounts for 6% of the molten salt mass, the reaction temperature is 1273K, the temperature fluctuation range is ±1K, and the gas flow rate is 200Ncm 3 min -1 , the reaction time is 7 hours, and the reaction is an intermittent operation.
[0032] ②Implementation results
[0033] Through the composition analysis of the product gas, it is found that the gas composition is mainly hydrogen (H 2 ) and carbon monoxide (CO), the molar ratio of the two is close to 2:1, the vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com