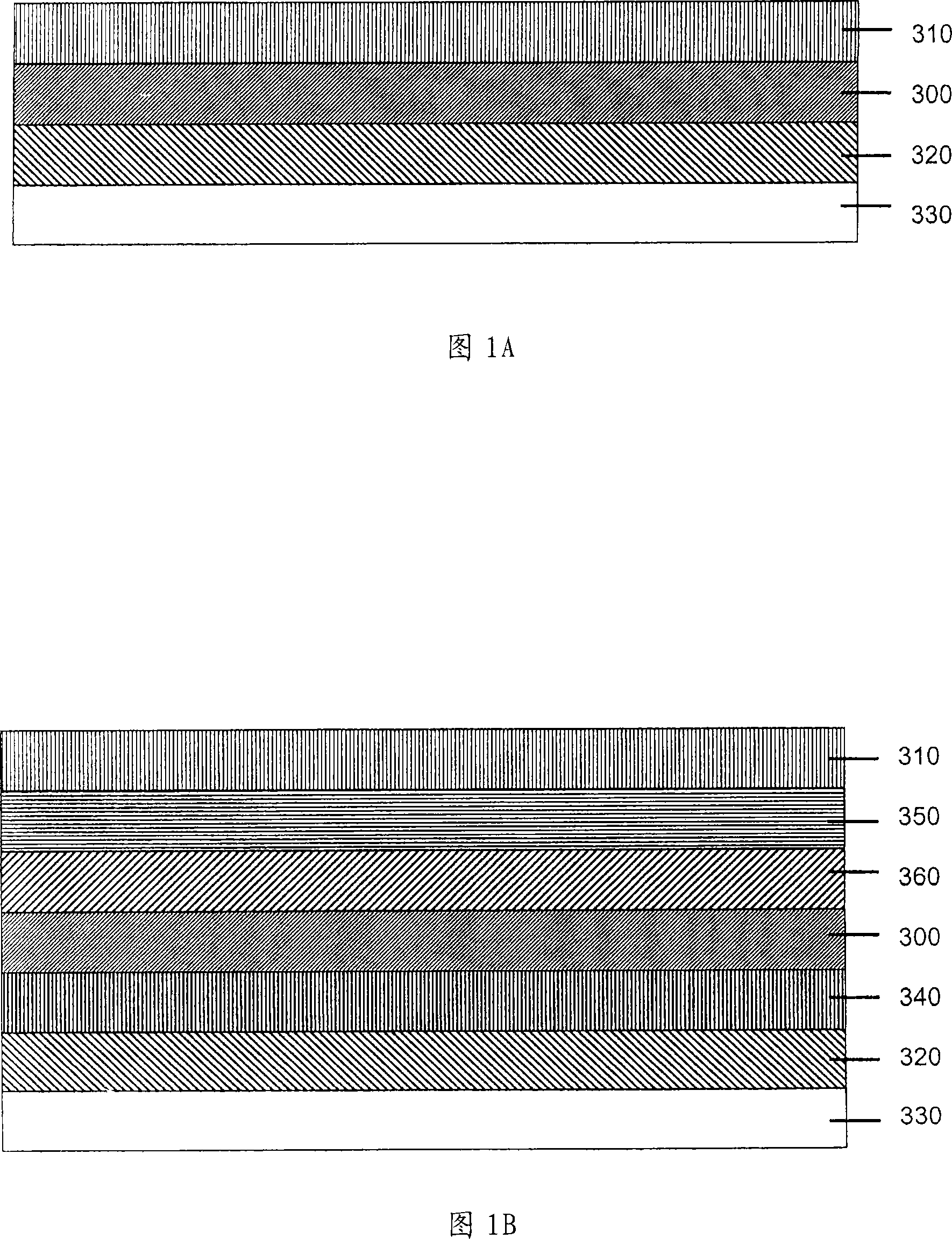
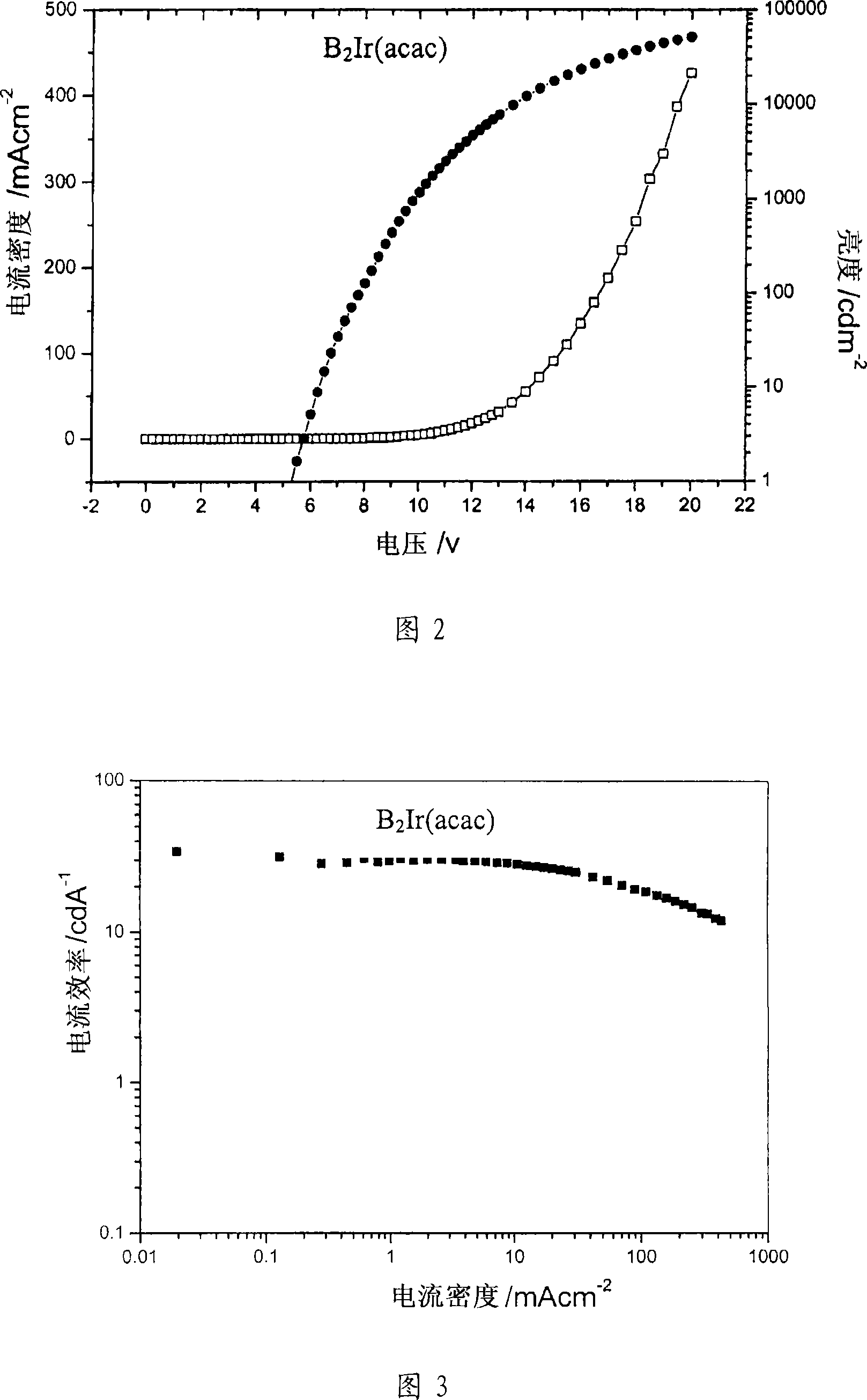
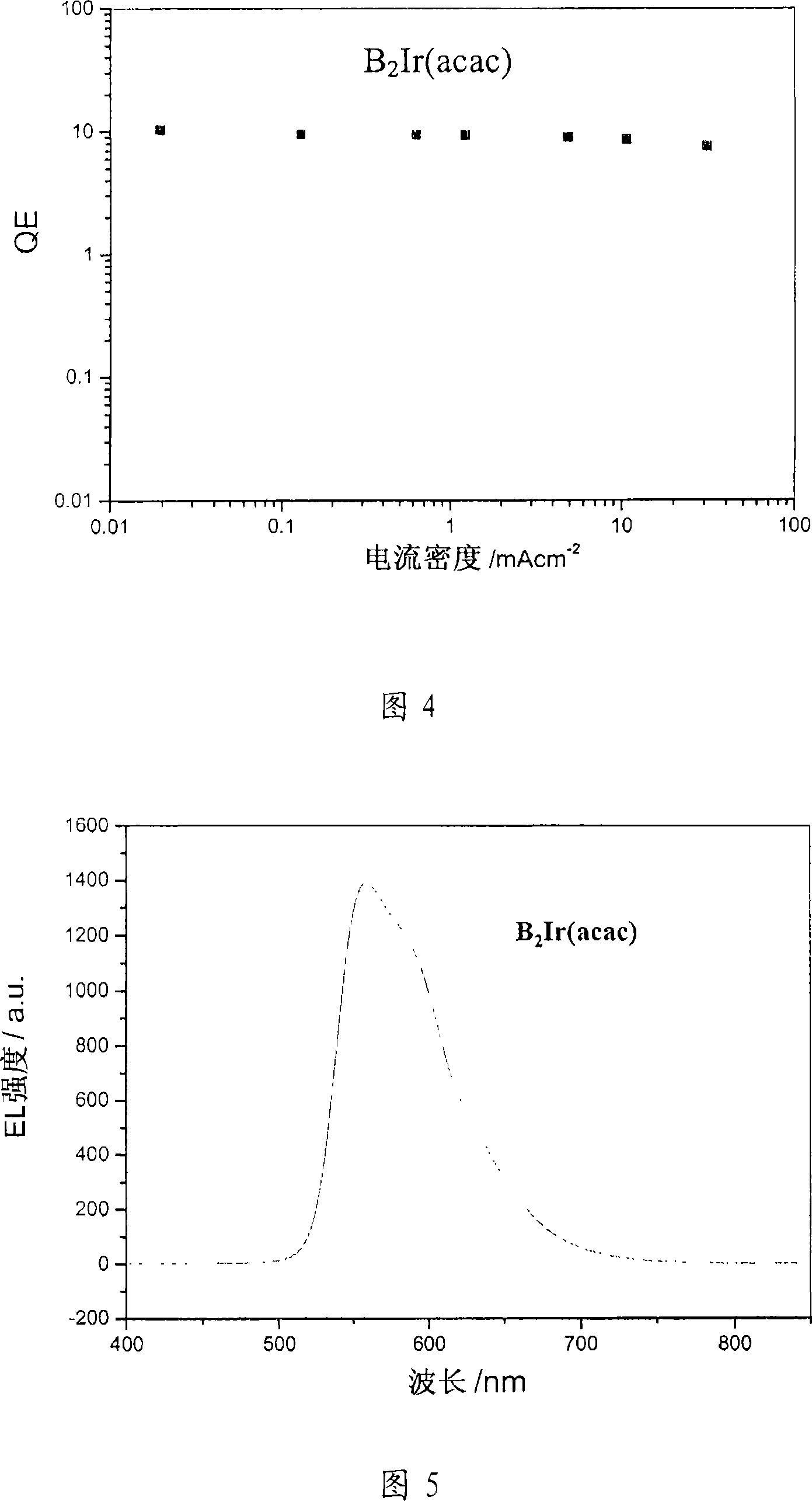
Solution processed organometallic complexes and their use in electroluminescent devices
An organometallic and complex technology, which can be used in electroluminescence light sources, indium organic compounds, platinum group organic compounds, etc., and can solve the problems of high-cost equipment and high cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Synthesis of 2-(trimethylsilyl)pyridine (compound 1)
[0136]
[0137] To 2-bromopyridine (4.74g, 0.030mol), CuI (0.14g, 0.74mmol and Pd (PPh 3 ) 2 Cl 2 (0.52g, 0.74mmol) in 100ml of diisopropylamine was added (trimethylsilyl)acetylene (3.0g, 0.030mol). The mixture was stirred at room temperature overnight under nitrogen atmosphere. After removing the solvent under reduced pressure, the residue was purified by distillation under reduced pressure to provide 5.0 g (95% yield) of pure compound 1 of 2-(trimethylsilyl)pyridine.
Embodiment 2
[0139] Synthesis of 2-(trimethylsilyl)-5-bromopyridine (compound 2)
[0140]
[0141] Add (trimethyl silyl)acetylene (1.47 g, 0.015 mol). The mixture was stirred at room temperature overnight under nitrogen atmosphere. After removing the solvent under reduced pressure, the residue was purified by a flash column to provide 3.45 g (90% yield) of compound 2 of 2-(trimethylsilyl)-5-bromopyridine.
Embodiment 3
[0143] Synthesis of 2-(2',3',4',5'-tetraphenyl)phenyl-5-bromopyridine (Compound 3)
[0144]
[0145] To a solution of 2-(trimethylsilyl)-5-bromopyridine (1.27 g, 5 mmol) in a mixture of THF and methanol was added 1 ml NaOH (5N). The reaction mixture was stirred at room temperature for 1 hour. Then 50 ml of ethyl acetate was added, the mixture was washed with water and brine and dried over anhydrous magnesium sulfate. After removal of the solvent, the residue was refluxed overnight with tetraphenylcyclopentadienone (2 g, 5.2 mmol) in 50 ml o-xylene. After cooling to room temperature, the solvent was removed by flash column and the residue was purified by recrystallization in ethanol 2-3 times to afford 2.17 g (81% yield) of pure 2-(2',3', 4',5'-tetraphenyl)phenyl-5-bromopyridine (Compound 3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com