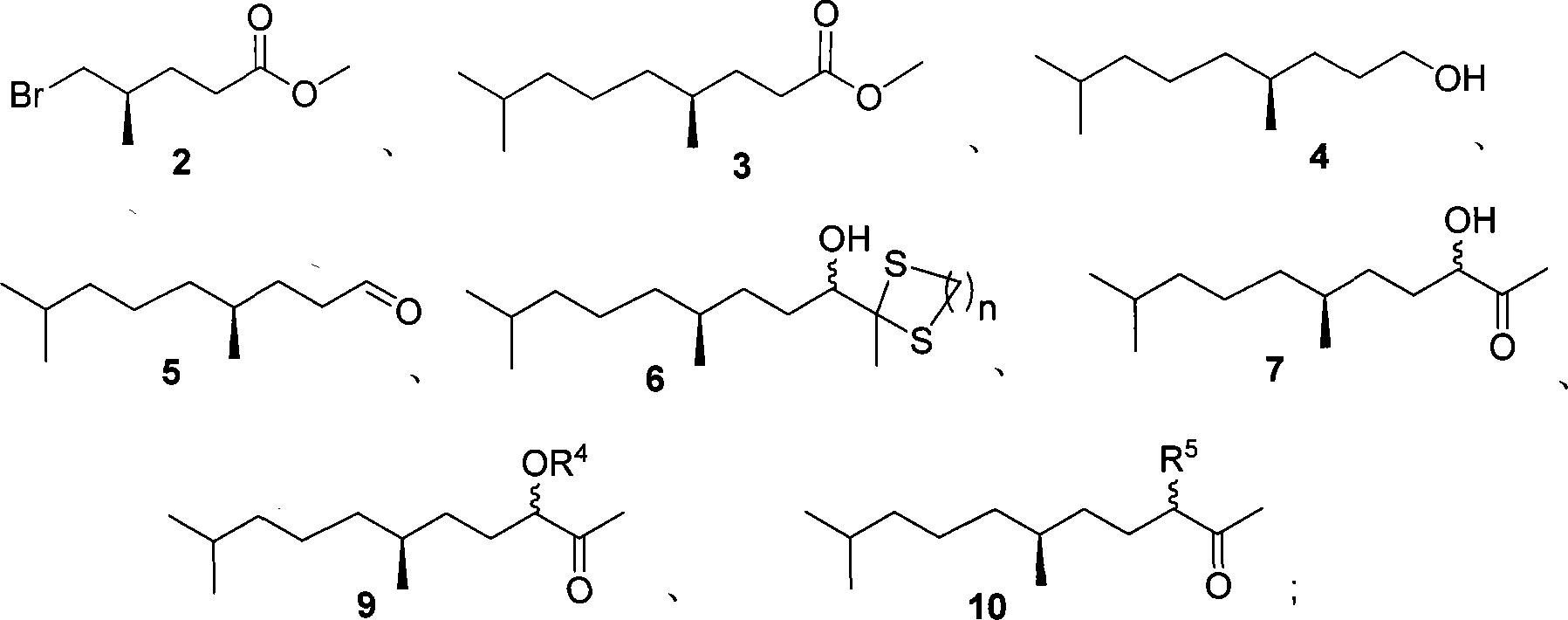
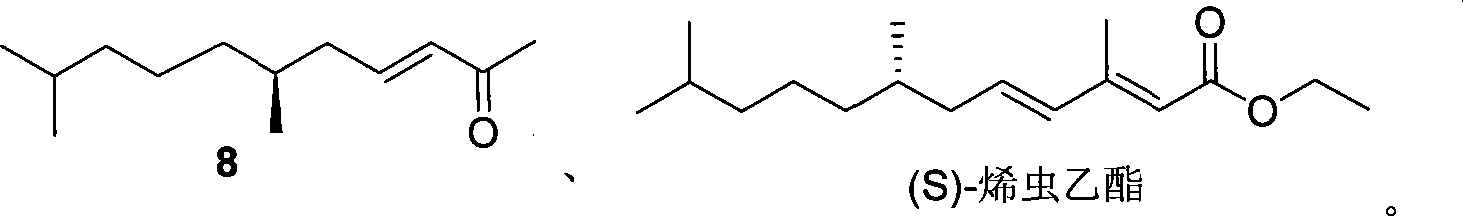
(6S)-6-methyl-2-ketone derivant, synthesizing method and use in synthesis of (S)-methoprene
A technology of methoprene and derivatives, which is applied in the field of -6-methyl-2-one derivatives and achieves the effects of high yield, short steps and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthesis of embodiment 1 (R)-5-bromo-4-methylpentanoic acid methyl ester (2)
[0037]
[0038] In a 500mL three-necked bottle, dissolve 50g of the crude product lactone 1 in 200mL of anhydrous methanol. Pass dry HBr gas under stirring, and keep white mist in the bottle. After reacting at room temperature for 16 hours, it was detected by TLC that the raw material disappeared, and the flow of HBr gas was stopped. The solution was spin-dried under reduced pressure, the residue was extracted with ether (4×100mL), and the organic phase was washed with saturated NaHCO 3 Solution (2×80 mL), washed with saturated brine (2×50 mL), dried over anhydrous sodium sulfate, and spin-dried under reduced pressure. Distilled under reduced pressure, 28.54 g of compound 2 was collected as a 52°C-54°C / 60Pa fraction, with a weight yield of 56%. [α] D 25 2.16 (c1.1, CH 3 Cl) 1 H NMR (300MHz, CDCl 3 ): δ3.68(s, 3H), 3.36-3.39(m, 2H), 2.35(t, 2H, J=7.5Hz), 1.75-1.90(m, 2H), 1.52-...
Embodiment 2
[0039] Synthesis of Example 2 Compound 3
[0040]
[0041] Add 360mg (15mmol) of magnesium chips into a 50mL three-necked bottle, vacuum-fill with nitrogen three times, and then add 4mL of anhydrous THF. 1.2 mL (1.51 g, 10 mmol) of isopentyl bromide was dissolved in 6 mL of anhydrous THF, and added dropwise to the above reaction flask. First add 10 drops dropwise and stir. After the reaction is triggered, continue to add dropwise, keep the reaction system slightly hot, continue stirring for 1 hour after the drop is complete, and let it stand for use.
[0042] Another 50mL three-neck bottle was taken and vacuum filled with nitrogen three times. Compound 2 (0.418g, 2.0mmol) was dissolved in 5mL of anhydrous THF, and 0.1mL of 1.0M Li 2 CuCl 4 Tetrahydrofuran solution, 0.96 mL NMP (991 mg, 10.0 mmol). At room temperature, 2.5 mL (2.5 mmol) of the above-prepared Grignard reagent was added dropwise, and after 3 hours of reaction, TLC detected that the raw material disappeared...
Embodiment 3
[0043] Synthesis of Example 3 Compound 4
[0044]
[0045] Lithium aluminum hydride (0.711g, 18.7mmol) was suspended in 20mL of anhydrous ether, and the ether solution (20mL) of 3 (3.12g, 15.6mmol) was added dropwise to the above system at 0°C. After the dropwise addition, it was raised to room temperature and reacted for 3 hours, and TLC detected that the reaction was complete. The reaction was carefully quenched with sodium sulfate containing crystal water until a loose solid was produced, which was filtered with suction and washed several times with diethyl ether. Spin-dried under reduced pressure, followed by flash column chromatography to obtain 42.58 g of a colorless oily substance with a yield of 96%. [α] D 26 -2.12 (c0.99, CHCl 3 ); 1 H NMR (CDCl 3 , 300Mz) δ: 3.63(t, J=6.6Hz, 2H), 1.67~1.09(m, 12H), 1.36(br s, 1H), 0.90~0.85(m, 9H); 13 C NMR (CDCl 3 , 75MHz) δ: 63.4, 39.3, 37.2, 32.9, 32.6, 30.2, 27.9, 24.7, 22.7, 22.6, 19.6; IR (film) v: 3328, 2956, 2929, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com