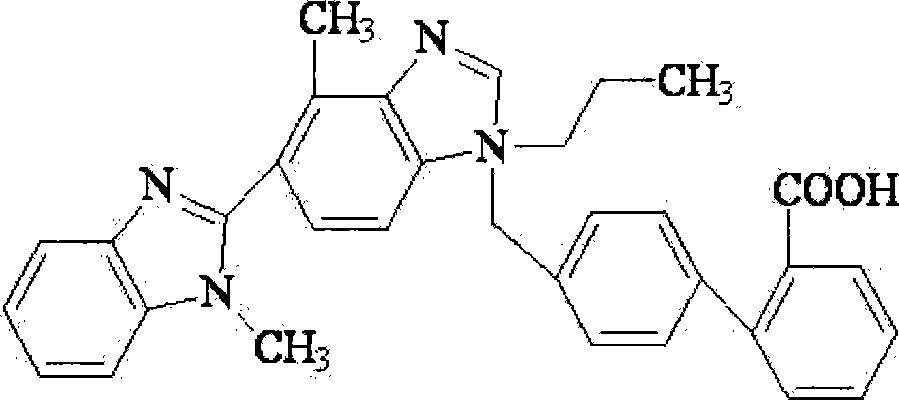
Compound preparations for treating hypertension and method for preparing the same
A technology for compound preparations and high blood pressure, which is applied to medical preparations containing active ingredients, pill delivery, and pharmaceutical formulations, etc. It can solve problems such as poor stability of tablet preparations, excessively strong antihypertensive synergistic effects, and impact on drug stability. , to achieve a reliable and safe step-down effect, reduce stability decline, and avoid the effect of stability decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The raw and auxiliary materials used to prepare 1000 tablets of the compound preparation in Example 1 of the present invention are shown in Table 6 below.
[0074] Table 6 The consumption of the embodiment of the present invention one 1000 compound preparation raw materials
[0075] Inner Chip Components
1000 tablets dosage
Core outer layer components
1000 tablets dosage
12.5g
40g
30g
146g
30g
3.36g
4g
Povidone K30
12g
40g
12g
0.8g
4.4g
[0076] Preparation:
[0077] 1. Inner chip preparation
[0078] Hydrochlorothiazide, lactose, microcrystalline cellulose, sodium carboxymethyl starch and starch were respectively passed through a ...
Embodiment 2
[0085] The raw and auxiliary materials used to prepare 1000 tablets of the compound preparation in Example 2 of the present invention are shown in Table 7 below.
[0086] Table 7 The amount of raw and auxiliary materials for 1000 tablets of compound preparations in Example 2 of the present invention
[0087] inner sheet components
1000 tablets dosage
Core outer layer components
1000 tablets dosage
12.5g
80g
30g
292g
30g
13.44g
4g
Povidone K30
24g
40g
24g
0.8g
8.8g
[0088] Preparation:
[0089] 1. Inner chip preparation
[0090] Hydrochlorothiazide, lactose, microcrystalline cellulose, sodium carboxymethyl starch and starch are respective...
Embodiment 3
[0096] Preparation:
[0097] 1. After the inner core was prepared according to the steps of Example 1, the inner core coated tablet was prepared according to the following method.
[0098] Preparation of coating slurry: Dissolve 16 g of Opadry (purchased from Shanghai Colorcon Coating Technology Co., Ltd.) in an appropriate amount of 70%-75% ethanol solution, fully swell for 45 minutes, stir to dissolve evenly, and set aside .
[0099] Coating operation: Put the pressed inner chip into the coating machine, start the hot air device and exhaust device, adjust the speed to 2-3r / min for preheating. When the temperature rises to 50-60°C, turn on the peristaltic pump, adjust the speed to 6-12r / min, control the inlet temperature at 90-100°C, and spray at a speed of 130-150g / min, spray coating. The flow rate and time during spraying are well controlled to prepare the inner core coated tablet.
[0100] 2. The preparation of core outer layer particles is the same as in Example 1.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com