Lithium sulphide battery and method of producing the same
A lithium sulfide and chemical power supply technology, applied in the manufacture of electrolyte batteries, electrode manufacturing, lithium batteries, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
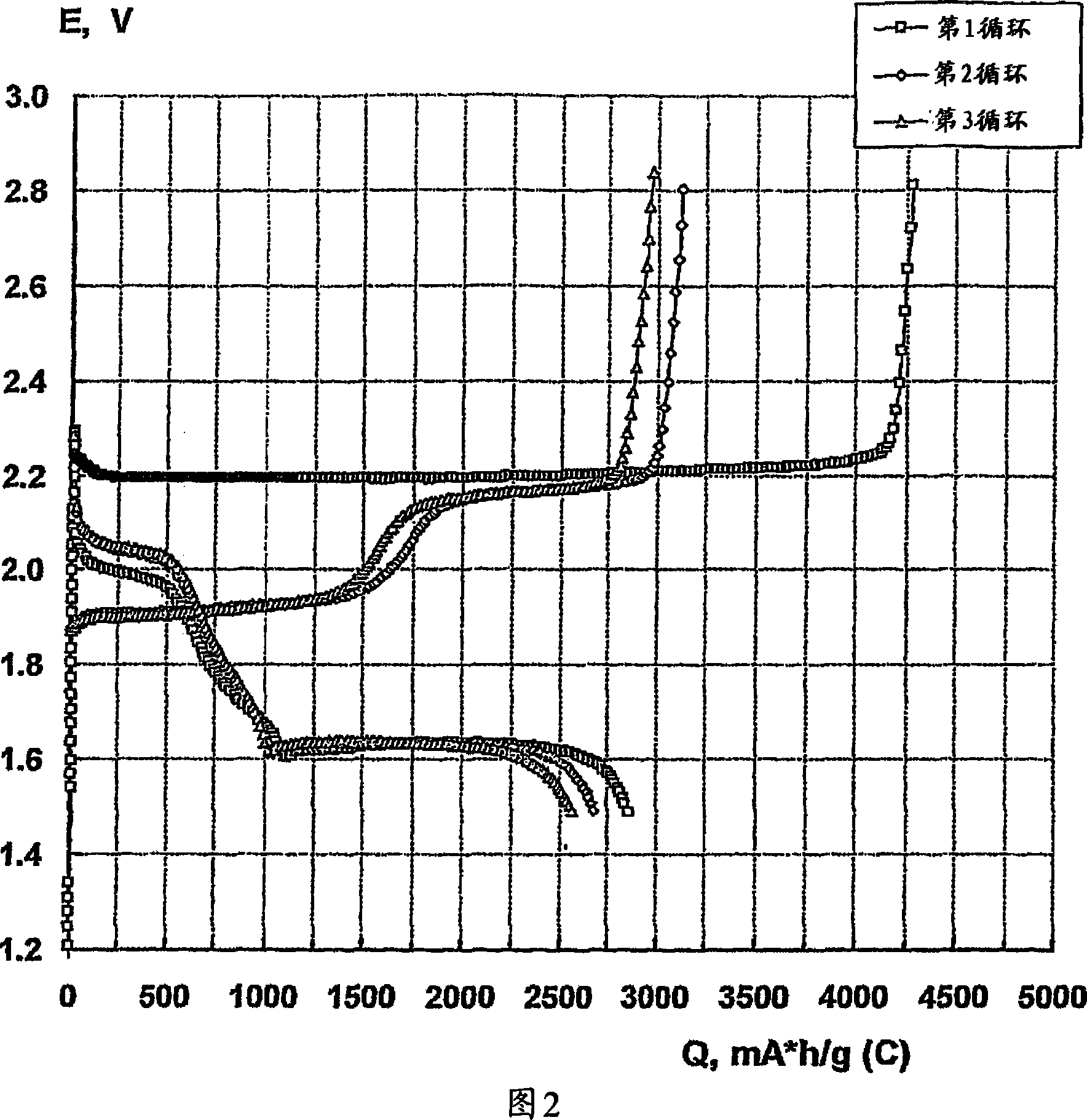
Image
Examples
Embodiment 1
[0062] Lithium sulfide, 98% (Sigma-Aldrich, UK) and sublimed sulfur, 99.5% (FisherScientific, UK) in a high-speed mill (Microtron MB550) at a mass ratio of 90:10 in an atmosphere of dry argon (moisture content 20 to 25 ppm) Grind down for 15 to 20 minutes. A ground mixture of lithium sulfide and sulfur was placed in a flask, and the electrolyte was added to the flask. A 1M solution of lithium trifluoromethanesulfonate (available from 3M Company, St. Paul, MN) in sulfolane (99.8%, GC standard, available from Sigma-Aldrich, UK) was used as the electrolyte. The mass ratio of liquid to solid is 10:1. The contents of the flask were mixed with a magnetic stirrer for 24 hours at room temperature. The liquid phase was separated from the insoluble solid phase by filtration. Analyze sulfur in the form of sulfides as well as total sulfur content. The total sulfur content in the initial electrolyte was also analyzed and considered.
[0063] Analysis results:
[0064] Total sulfur co...
Embodiment 2
[0074] An electrolyte solution of polysulfides (1M sulfolane solution of lithium trifluoromethanesulfonate) was prepared as described in Example 1, and the total sulfur content and sulfides were analyzed by chemical methods. Li 2 The mass ratio of S to S is 50:50.
[0075] Analysis results:
[0076] Total sulfur content in initial electrolyte, % mass ratio 25.8±0.1
[0077] In the electrolyte after reacting with a mixture of sulfur and lithium sulfide
[0078] Total sulfur content, % 31.8±0.1
[0079] In the electrolyte after reacting with a mixture of sulfur and lithium sulfide
[0080] Sulfide sulfur content, % 0.96±0.05
[0081] The composition and concentration of lithium polysulfide in the electrolyte after reacting with the mixture of sulfur and lithium sulfide were calculated by analyzing the results.
[0082] Calculation results:
[0083] Polysulfide Composition: Li 2 S 6.25
[0084] Concentration: 0.96%
Embodiment 3
[0086] An electrolyte solution of polysulfides (1M sulfolane solution of lithium trifluoromethanesulfonate) was prepared as described in Example 1, and the total sulfur content and sulfides were analyzed by chemical methods. Li 2 The mass ratio of S to S is 10:90.
[0087] analysis results;
[0088] Total sulfur content in initial electrolyte, % mass ratio 25.8±0.1
[0089] In the electrolyte after reacting with a mixture of sulfur and lithium sulfide
[0090] Total sulfur content, % 29.9
[0091] In the electrolyte after reacting with a mixture of sulfur and lithium sulfide
[0092] Sulfur content of sulfide, % 0.7
[0093] The composition and concentration of lithium polysulfide in the electrolyte after reacting with the mixture of sulfur and lithium sulfide were calculated by analyzing the results.
[0094] Calculation results:
[0095] Polysulfide Composition: Li 2 S 5.86
[0096] Concentration: 0.7%
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com