Glutamine transaminase zymogen gene for streptomyces hygroscopicus and expression thereof
A technology of transglutaminase proenzyme and transglutaminase enzyme, which is applied in the field of transglutaminase proenzyme and can solve the problem of low enzyme activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: Obtaining of the transglutaminase gene of Streptomyces hygroscopicus
[0052] The complete amino acid sequence of transglutaminase from Streptomyces platensis, Streptomyces mobaraensis, Streptomyces cinnamoneus and Streptomyces fradiae was found through the NCBI gene bank. According to the results reported in the literature, the starting sequence and termination sequence of the zymogen were found, and then the corresponding base sequences were compared; on the other hand, after the zymogen from natural Streptomyces hygroscopicus was purified, the N-terminal amino acid sequencing was performed.
[0053] Determine the primers for PCR as follows:
[0054] P1: 5'-GCTAGCGGTGACGACGAGG-3'
[0055] P2: 5'-TTACGGCCAGCCCTGCTTTAC-3'
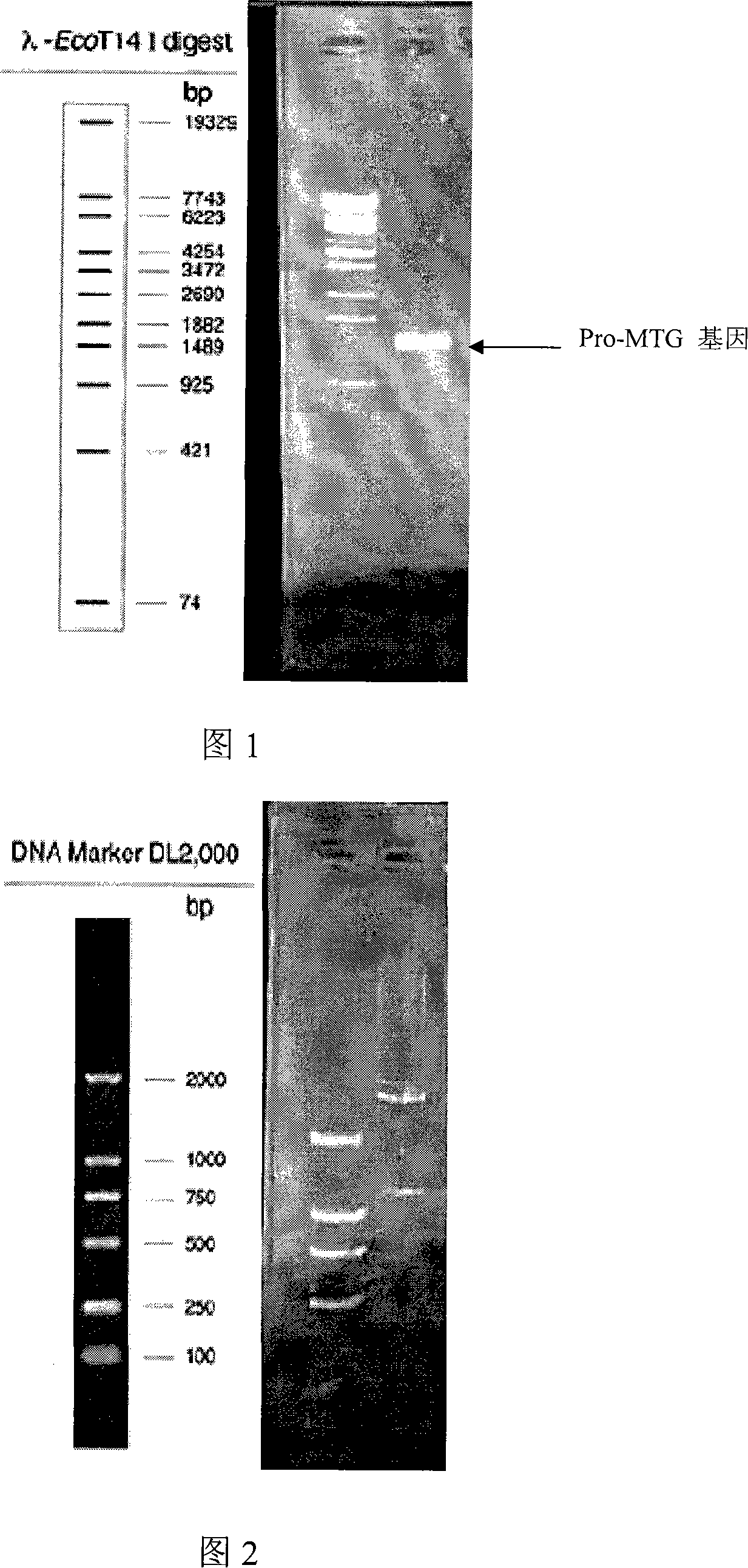
[0056] Using the above primers, using the extracted total DNA of Streptomyces hygroscopicus CCTCC M203062 as a template, the PCR reaction was carried out in a 50 μL system, and the reaction conditions were denaturation at 95°C for 5 m...
Embodiment 2
[0057] Embodiment 2: Cloning of transglutaminase progenase gene
[0058] The amplified DNA was recovered and purified using Shanghai Sangon UNIQ-10 Column Gel Recovery Kit, 4.5 μL of the purified product and 0.5 μL of the pMD-18T vector were operated according to the kit, connected at 16°C for 12 hours, and the ligated product was heat-shocked Escherichia coli JM109 was transformed. The specific operation is as follows:
[0059] 1) Take the JM109 competent cells out of the -80°C refrigerator and put them on ice quickly, and let them dissolve for 5 minutes.
[0060] 2) 10 μL of the ligation product was added to 100 μL of JM109 competent cells, and placed in ice for 30 minutes.
[0061] 3) After heating at 42°C for 90 seconds, place in ice for 3 minutes.
[0062] 4) Add 950 μL of SOC (preheated at 37° C.) culture medium, and culture with shaking at 37° C. for 90 minutes.
[0063] 5) In the presence of 0.04mg / mL X-Gal (5-bromo-4-chloro-3-indole-α-D-galactoside), 0.024mg / mL IP...
Embodiment 3
[0065] Embodiment 3: Expression of Streptomyces hygroscopicus transglutaminase
[0066] Use restriction endonucleases NcoI and BamHI to cut off from pMD / pro-MTG, and then cut the expression vector pET20b in the same way, then use T4 DNA ligase to carry out ligation reaction, and transform the ligated product into Escherichia coli JM109 first, After obtaining the correct expression plasmid pET / pro-MTG, the expression plasmid was transformed into BL21(DE3) or Rosetta(DE3)pLysS. The engineered bacteria containing the expression plasmid pET / pro-MTG were named BL21 / pET-MTG or Rosetta / pET-MTG.
[0067] To induce expression proceed as follows:
[0068](1) Quickly add 0.1ml of BL21 / pET-MTG or Rosetta / pET-MTG bacterial solution preserved in glycerol tube into a 50ml Erlenmeyer flask containing 25ml of LB medium containing 0.1mg / mL Amp, and cultivate the seeds at 200rpm overnight ( 12h).
[0069] (2) The seeds were inoculated into three 250ml Erlenmeyer flasks containing 50ml of LB m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com