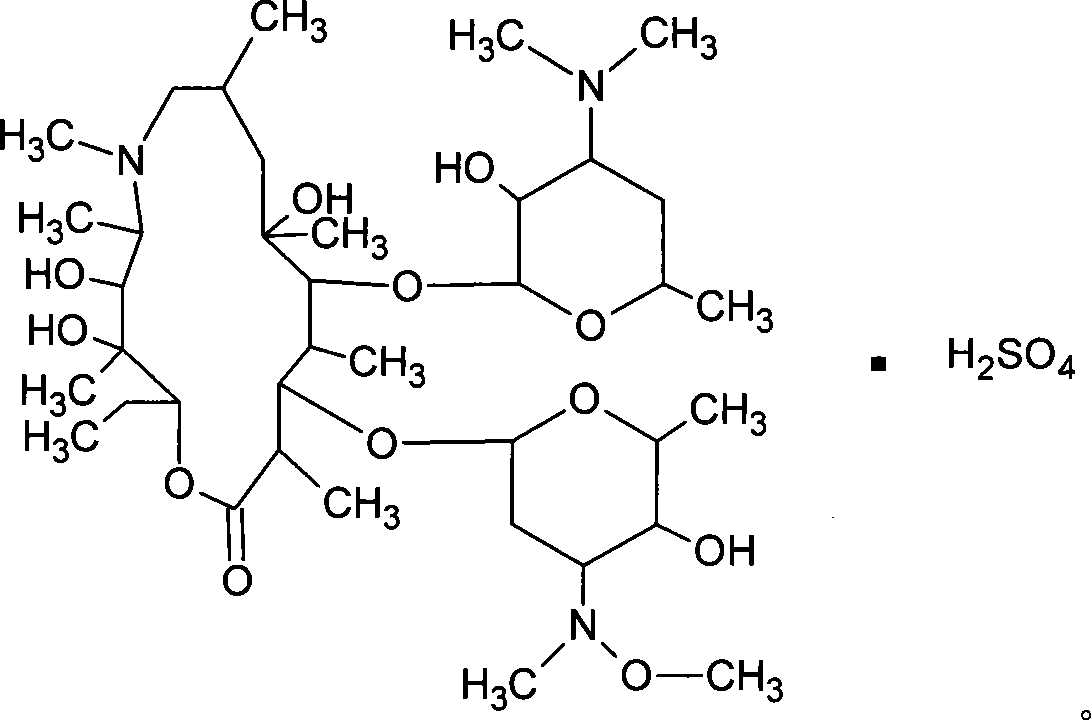
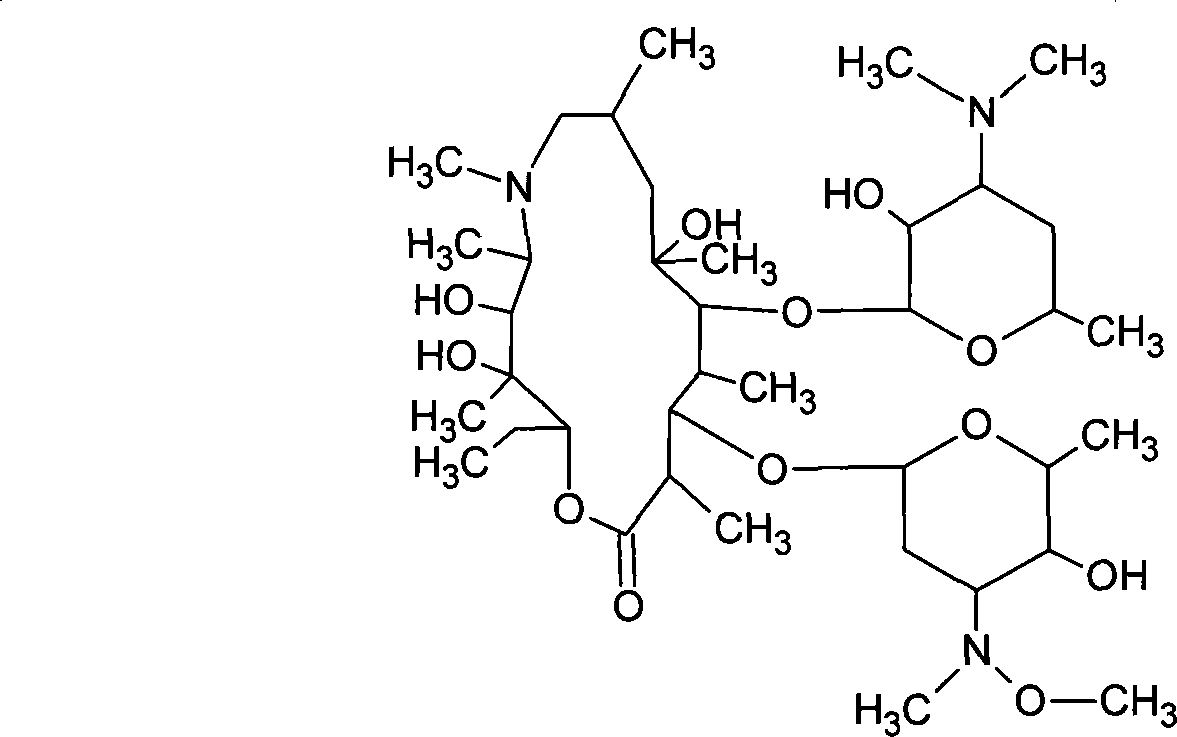
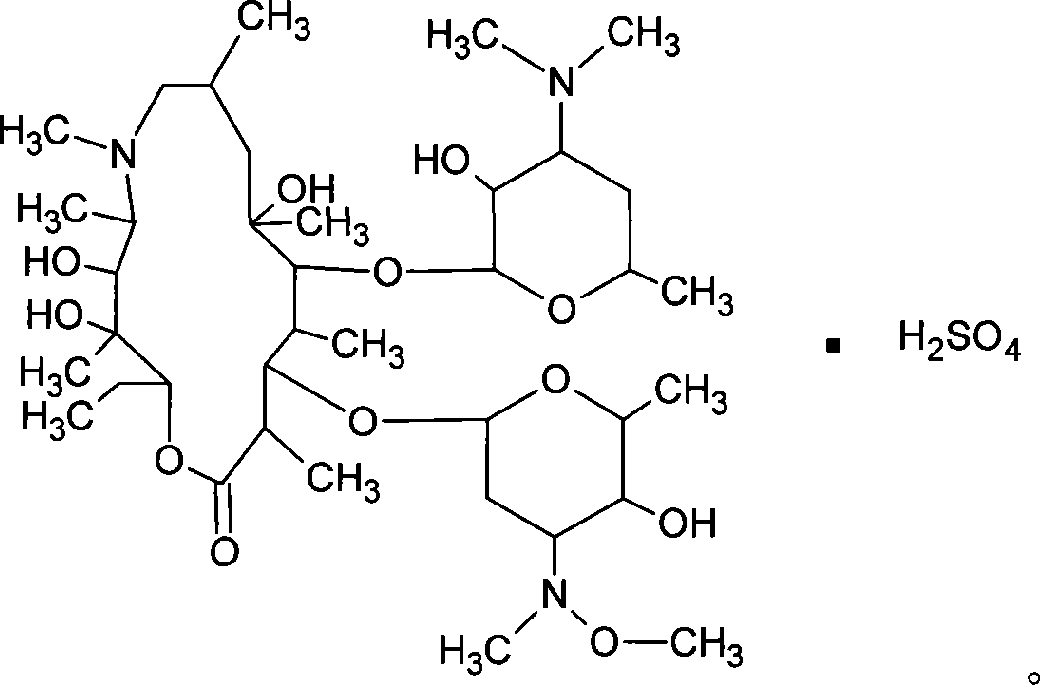
Sulfuric acid azithromycin, application thereof, freeze dried of the sulfuric acid azithromycin and preparation method for the freeze dried
A technology of freeze-dried powder injection and azithromycin, which is applied in the field of medicine, can solve the problems of gastrointestinal adverse reactions and low bioavailability, and achieve the effect of ensuring safety and convenience, good appearance and good shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Weigh 25kg of absolute ethanol, weigh 12.5kg of azithromycin, add to a 100L salt forming tank, stir to completely dissolve, slowly add 4.05kg of anhydrous sodium bisulfate to react for 90 minutes at below 5°C, add 0.25kg of activated carbon, 2kg of sulfuric acid Sodium decolorization and dehydration for 30 minutes, discharge, decarbonization and pressure filtration, and fine filtration at 0.8um and 0.22um to a crystallization tank equipped with 85.8kg of anhydrous ether after depyrogenation, decarbonization, fine filtration and sterilization filtration. Stir and crystallize for 30 minutes, filter with suction, wash with anhydrous ether, drain, transfer to a double-cone dryer, dry at about 60°C, and pack. The weight yield of fine product is 97.8%.
Embodiment 2
[0042]Weigh 250g of absolute ethanol, weigh 125g of azithromycin, add to a 1000ml three-necked bottle, stir to completely dissolve, slowly add 400g of anhydrous sodium bisulfate to react for 90 minutes below 5°C, add 25g of activated carbon, 200g of sodium sulfate for decolorization, Dehydration for 30 minutes, fine filtration at 0.8um and 0.22um, pour into a three-necked bottle filled with 858g of anhydrous ether, stir and crystallize for 30 minutes, filter with suction, wash with anhydrous ether, drain, transfer to a vacuum oven, 60 Dry at about ℃ and pack. The weight yield of the refined product is 101.0%.
Embodiment 3
[0044] Weigh 250g of absolute ethanol, weigh 125g of azithromycin, add it into a 1000ml three-necked bottle, stir to completely dissolve, slowly add 300g of anhydrous sodium bisulfate and react for 90 minutes below 5°C, add 25g of activated carbon, 200g of sodium sulfate for decolorization, Dehydration for 30 minutes, fine filtration at 0.8um and 0.22um, pour into a three-necked bottle filled with 858g of anhydrous ether, stir and crystallize for 30 minutes, filter with suction, wash with anhydrous ether, drain, transfer to a vacuum oven, 60 Dry at about ℃ and pack. The weight yield of fine product is 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com