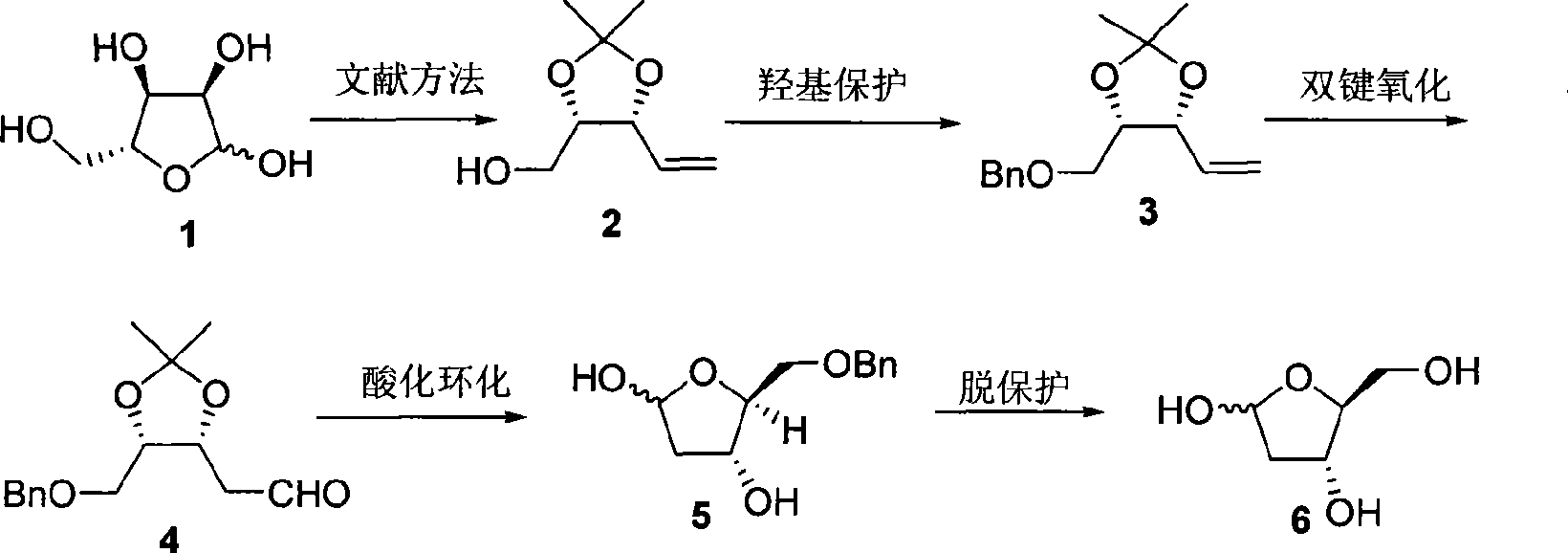
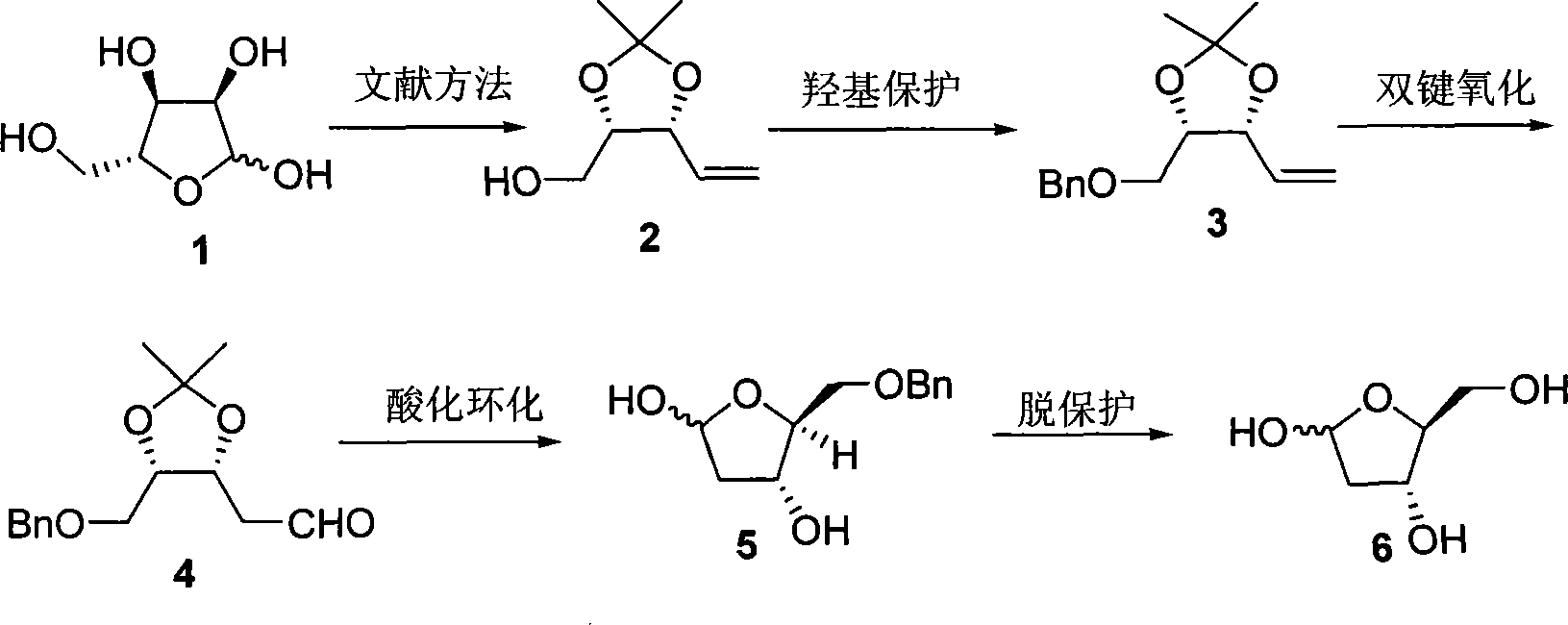
Method of preparing 2-deoxy-L-ribose
A technology of ribose and equation, which is applied in the field of preparation of 2-deoxy-L-ribose, can solve the problems of limited source of raw materials, difficulty in large-scale production, long synthetic route, etc., and achieves easy-to-obtain raw materials, mild conditions and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] First prepare (2) from D-(-)-ribose according to the literature method (V. Jaeger, B. Hafele, Synthesis, 1987, 9, 801), and then prepare 2-deoxy-L-ribose according to the following steps.
[0027] In a 25mL round bottom flask, weigh NaH (334mg, 8.34mmol), add anhydrous THF (11.0mL), cool the reaction system to 0°C, add dropwise (2) (584mg, 3.69mmol) dissolved in anhydrous THF (3.0mL) the formed solution, after addition, stirred at 0°C for 30min, added Bu 4 NI (51mg, 0.14mmol), BnBr (1.1mL, 9.04mmol) was added dropwise, the temperature was gradually raised to room temperature and stirring was continued for 18h, then water (5.0mL) was added, the organic phase was separated, and the aqueous phase was extracted with diethyl ether (3×5mL ). The organic phases were combined, dried over anhydrous magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure. The obtained crude product was purified by flash silica gel column chromatography to obtain (3)...
Embodiment 2
[0032] Similar to Example 1, the difference is that the amount of sodium hydride in the preparation (3) step is 1.8 molar multiples of (2), Bu 4 The consumption of NI is 10% mole; The consumption of palladium chloride and cuprous chloride is respectively 0.1 and 1.0 mole multiples of (3) in the preparation (4) step, and the ratio of the two in the mixed solvent of DMF and water is 6: 2. The reaction temperature is 75°C and the reaction time is 12h; the concentration of (4) in tetrahydrofuran in the step (5) of preparing benzyl 2-deoxy-L-ribose is 0.15mol / L; preparing 2-deoxy-L-ribose (6) The amount of palladium carbon in the step is 0.2 mole multiple of (5), the concentration of (5) in methanol solution of 10% formic acid is 0.35mol / L, and the reaction time is 4h.
Embodiment 3
[0034] Similar to Example 1, the difference is that the concentration of (2) added in the preparation (3) step is 0.6mol / L; the consumption of palladium chloride and cuprous chloride in the preparation (4) step is respectively 0.2 of (3) and 1.3 molar multiples, the ratio of the two in the mixed solvent of DMF and water is 6: 2, (3) the concentration in DMF and water solvent is 0.7mol / L, the reaction temperature is 75 ℃, and the reaction time is 12h; the preparation of benzyl The concentration of (4) in tetrahydrofuran in the base 2-deoxy-L-ribose (5) step is 0.15mol / L, and the consumption of palladium carbon in the preparation of 2-deoxy-L-ribose (6) step is 0.2% of (5). Molar multiple, (5) The concentration in methanol solution of 10% formic acid is 0.35mol / L, and the reaction time is 4h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com