Method for separating hemporfin isomer and separated isomer
A technology of isomer and volume ratio, applied in the field of photosensitizers for photodynamic therapy, can solve problems such as single molecular structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
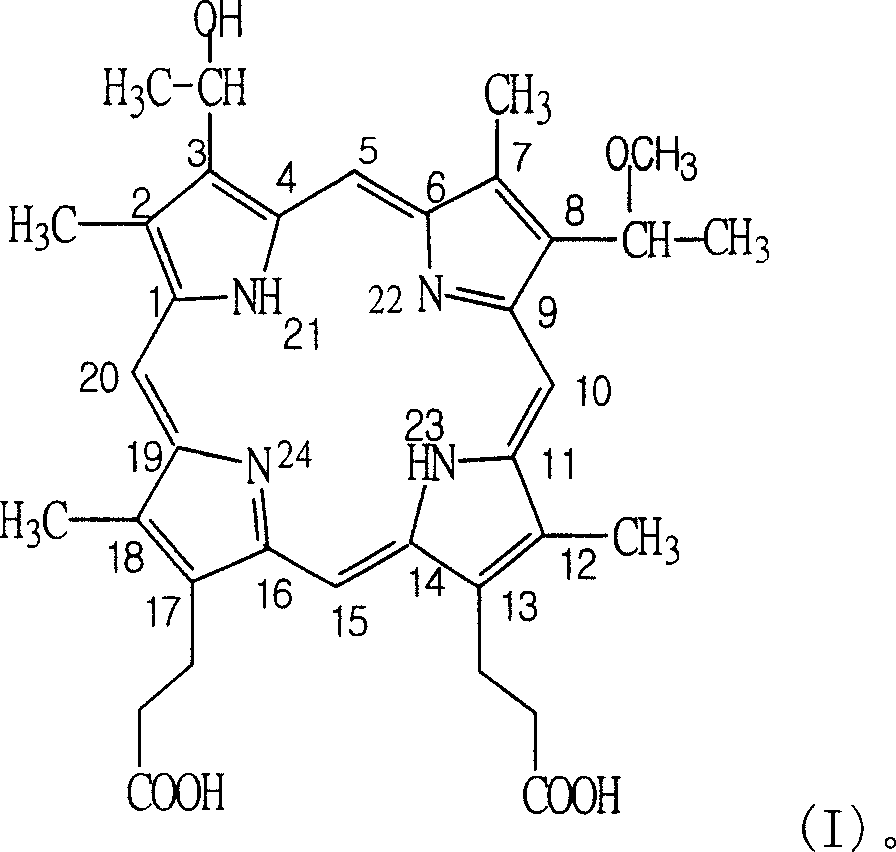
[0075] Embodiment 1: the preparation of hemporfin
[0076] 1.3 (8)-(1-methoxyethyl)-8(3)-(1-hydroxyethyl) hypoporphyrin IX crude product preparation
[0077] In a 50L acid-resistant glass-lined reaction pot, add 30L of methanol / water mixture with a volume ratio of 3:1, slowly drop it in from a liquid dropper under stirring, and prepare 3 according to the method described in Example 1 of the Chinese patent 01105208.2 specification , 8-bis-(1-bromoethyl)-hypoporphyrin IX hydrobromide glacial acetic acid saturated solution 10L, control the rate of addition to keep the temperature of the reaction mixture at 10-20°C. After the dropwise addition, the reaction solution continued to stir for 2h and left for 4h. Then add 10N NaOH aqueous solution dropwise under stirring until the reaction liquid is strongly alkaline (pH13 or so), let stand for more than 10h, add acetic acid to neutralize to pH4~5, add 5 times the volume of water to dilute, stand overnight, the precipitate will natural...
Embodiment 2
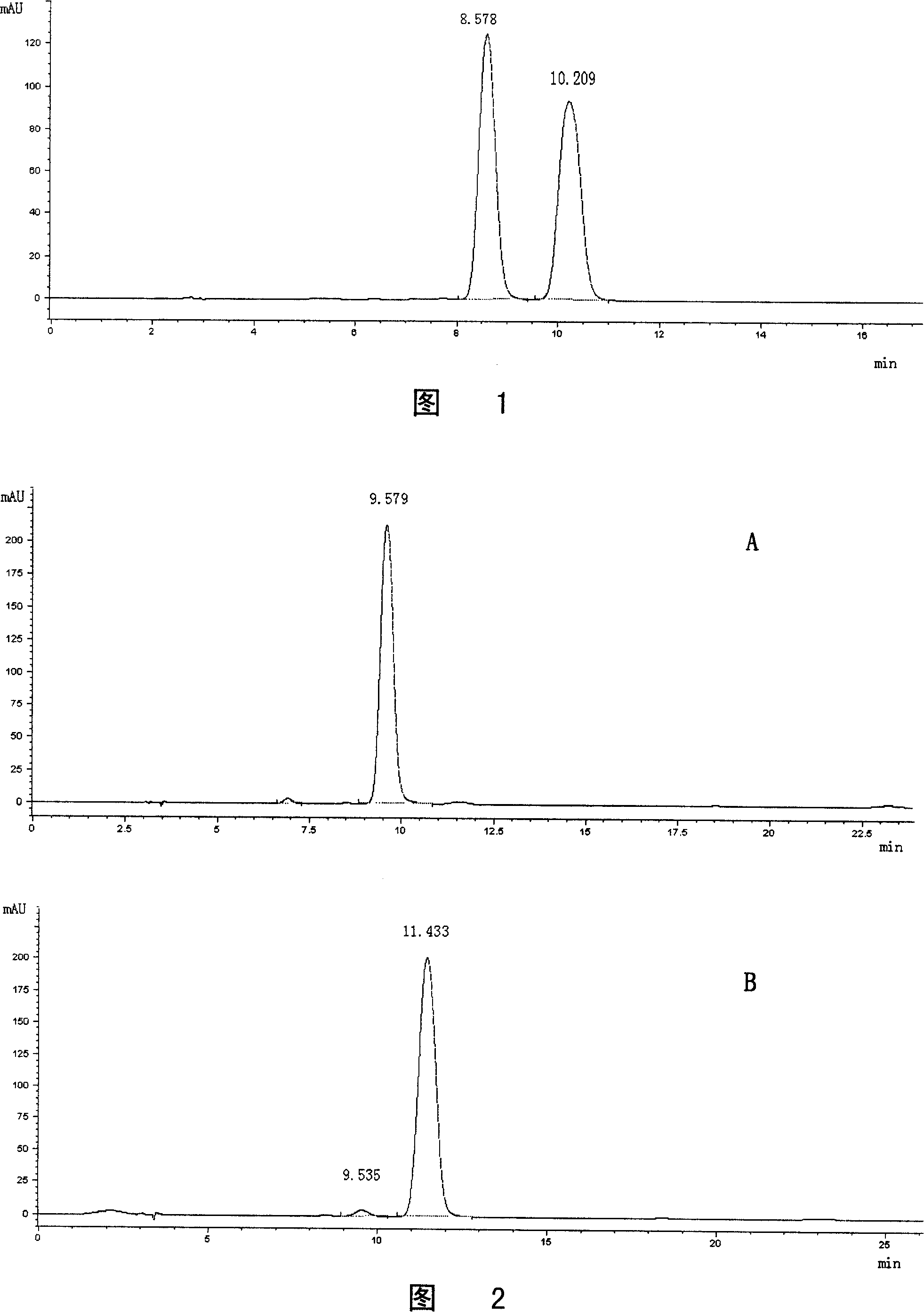
[0079] Example 2: HPLC Separation of Hemporfin Positional Isomers
[0080] Instrument: Agilent 1100 high performance liquid chromatography; DAD detector, detection wavelength: 395nm
[0081] Mobile phase: methanol-1.5M acetic acid-ammonium acetate buffer (pH4.76) (65:35V / V)
[0082] Column: MOS-Hypersil (C 8 ), 10×250mm
[0083] Flow rate: 2mL / min
[0084] Column pressure: 125 Bar
[0085] Take 100 mg of the hemporfin sample prepared in Example 1 above, dissolve it in HPLC mobile phase, prepare a sample solution with a concentration of 5 mg / mL, and filter it before injection. 400 microliters of the above-mentioned solutions were injected each time. After the injection, Fraction A was collected respectively according to the HPLC chromatogram (see Figure 1), the retention time was 11.94 minutes and the retention time of Fraction B was 14.35 minutes. Accumulated 40 times, a total of 136ml of fraction A and 133ml of fraction B were collected. Pour the collected solution into...
Embodiment 3
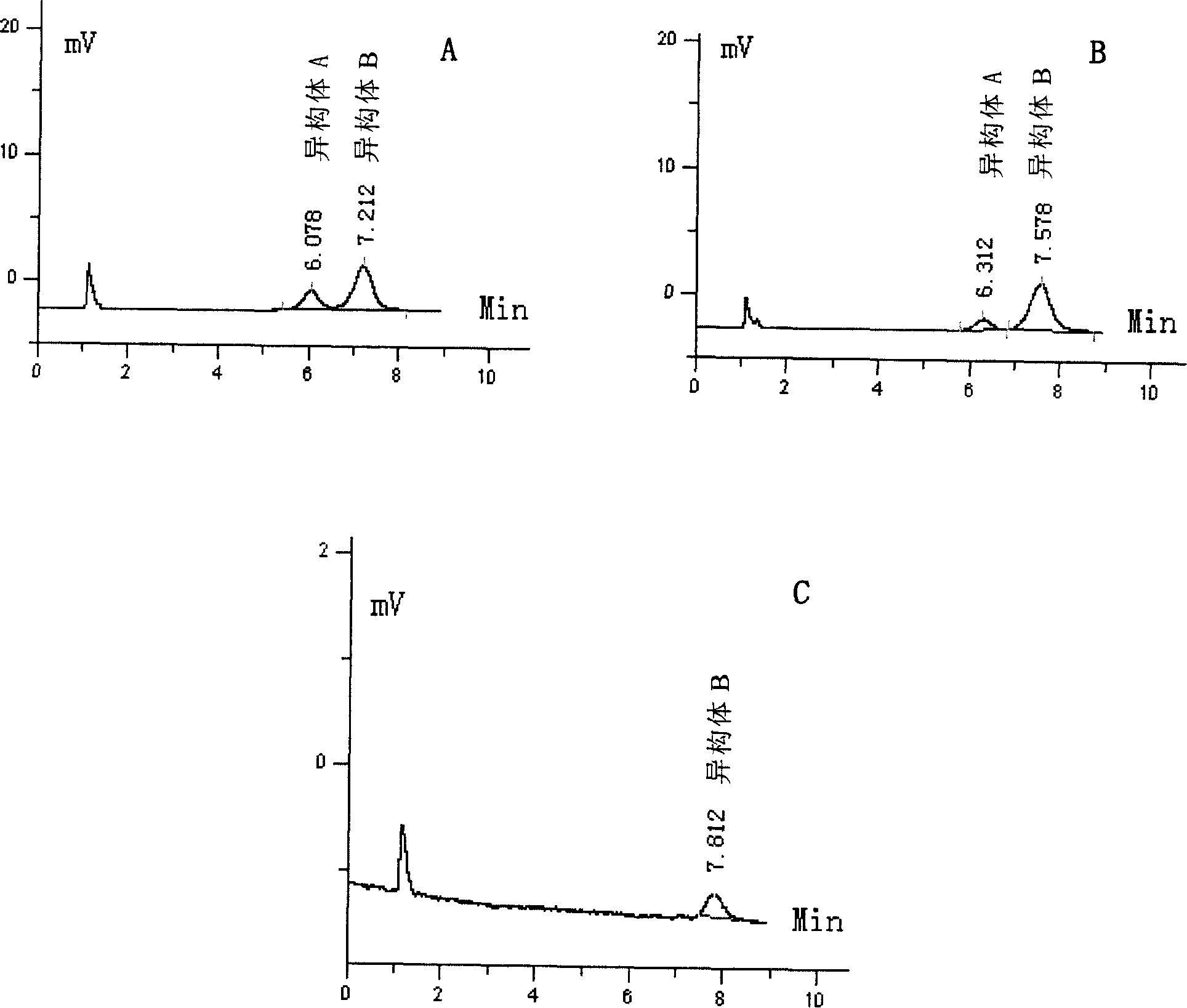
[0089] Example 3: Preparative Separation of Hemporfin Positional Isomers
[0090] The separation system adopts Flash150M with built-in prepacked C8 chromatographic column (150mm ID×30cm), produced by Biotage Company (USA), the silica gel particle size is 35-70 μm, and the pore size is 60A.
[0091] Sample preparation: get 3 (or 8)-(1-methoxyethyl)-8 (or 3)-(1-hydroxyethyl) hypoporphyrin IX pure product (content>95%) prepared in Example 1 30 g was dissolved in tetrahydrofuran and filtered, and 100 g of C8 reversed-phase silica gel was added, concentrated under reduced pressure, evaporated to dryness, and added to the loading column SIM for sample loading.
[0092] Sample loading and collection: Use methanol-1.5M acetic acid-ammonium acetate buffer (pH4.76) (65:35V / V) as the mobile phase, pass the above sample through the loading column SIM, and bring it into the chromatographic column for separation , the flow rate is controlled at 200ml / min, the detector wavelength is 395nm, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com