Medicine lipid composition
A composition and substance technology, applied in the field of active agent delivery system, amphiphilic composition and preparation, can solve the problems such as difficult to provide dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1 - Non-Layered Inverse Phase Nanoparticles
[0136] 1.1 - Preparation of non-lamellar dispersions
[0137] Non-lamellar (>80 wt% amphiphile) and lamellar (amphiphile <20wt%) particle dispersion. The components were mixed at the molecular level by heating at 70°C for 5 minutes and vortexing. The molten homogeneous lipid (2.012 g) was added dropwise to 38.01 g deionized water. The resulting crude dispersion was placed on a shaking table (350 rpm) and shaken for 24 hours to obtain a cloudy homogeneous dispersion.
[0138] Particle size was measured using laser diffraction (Coulter LS230). The size distribution was found to be narrow and unimodal with an average particle size of 95 nm.
[0139] 1.2-Heat treatment
[0140] An optional heat treatment cycle was carried out on the dispersion prepared in Example 1.1.
[0141] A sample (25 mL) of the dispersion produced in Example 1.1 was autoclaved (125°C, 20 minutes) and cooled to room temperature. The particle...
Embodiment 2
[0147] Example 2 - Other Compositions
[0148] A second composition was prepared by the method of Examples 1.1 and 1.2 to evaluate the effect of adding a higher concentration of stabilizer. Solutions of SPC and GDO (40 / 60 wt / wt) (2.017 g) and P80 (0.514 g) were molecularly mixed by heating at 70°C for 5 minutes and vortexing. The molten homogeneous lipid (2.006 g) was added dropwise to 38.00 g deionized water. The resulting crude dispersion was placed on a shaking table and shaken for 24 hours to obtain a cloudy homogeneous dispersion. Thereafter the dispersion was heat treated according to Example 1.2.
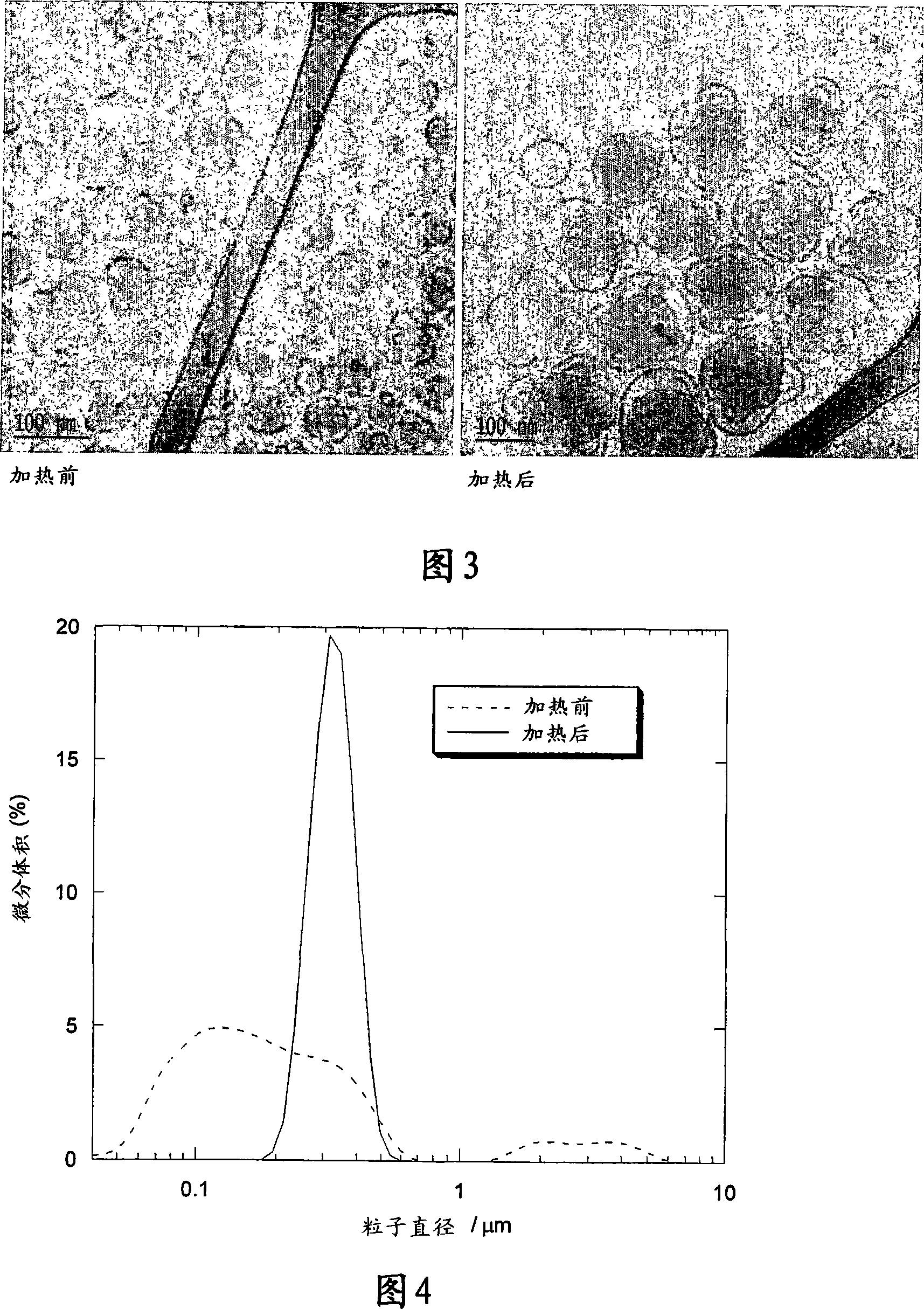
[0149] The size distributions before and after heat treatment were found to be narrow and unimodal with mean particle sizes of 88 and 129 nm, respectively. As shown in Figure 2, heat treatment also narrowed the particle size distribution. Figure 3 shows cryo-TEM pictures taken from samples before and after heat treatment. The cryo-TEM results clearly demonstrate the form...
Embodiment 3
[0156] Example 3 - Other Compositions
[0157] Another composition was prepared by the method of Examples 1.1 and 1.2 to evaluate the effect of adding another type of stabilizer. SPC and GDO (40 / 60wt / wt) (2.004g) and Solutol were mixed at the molecular level by heating at 70°C for 5 minutes and vortexing Solution of HS 15 (0.516 g). The molten homogeneous lipid (2.042 g) was added dropwise to 38.00 g deionized water. The resulting coarse dispersion was placed on a shaking table and shaken for 24 hours to obtain a cloudy dispersion containing some weakly dispersed macroscopic particles. In order to obtain a uniform dispersion, the samples were homogenized using a Microfluidizer at 5000 PSI and room temperature. The sample was passed through the homogenizer 5 times to obtain a milky homogeneous dispersion. Thereafter the dispersion was heat treated according to Example 1.2.
[0158] Figure 4 shows the size distribution obtained before and after heat treatment, showing tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com