Phthalazines derivatives, preparation method thereof, medicament composition and use
A derivative, phthalazine technology, applied in the fields of new phthalazine derivatives and their preparation methods, pharmaceutical compositions and uses, to achieve high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
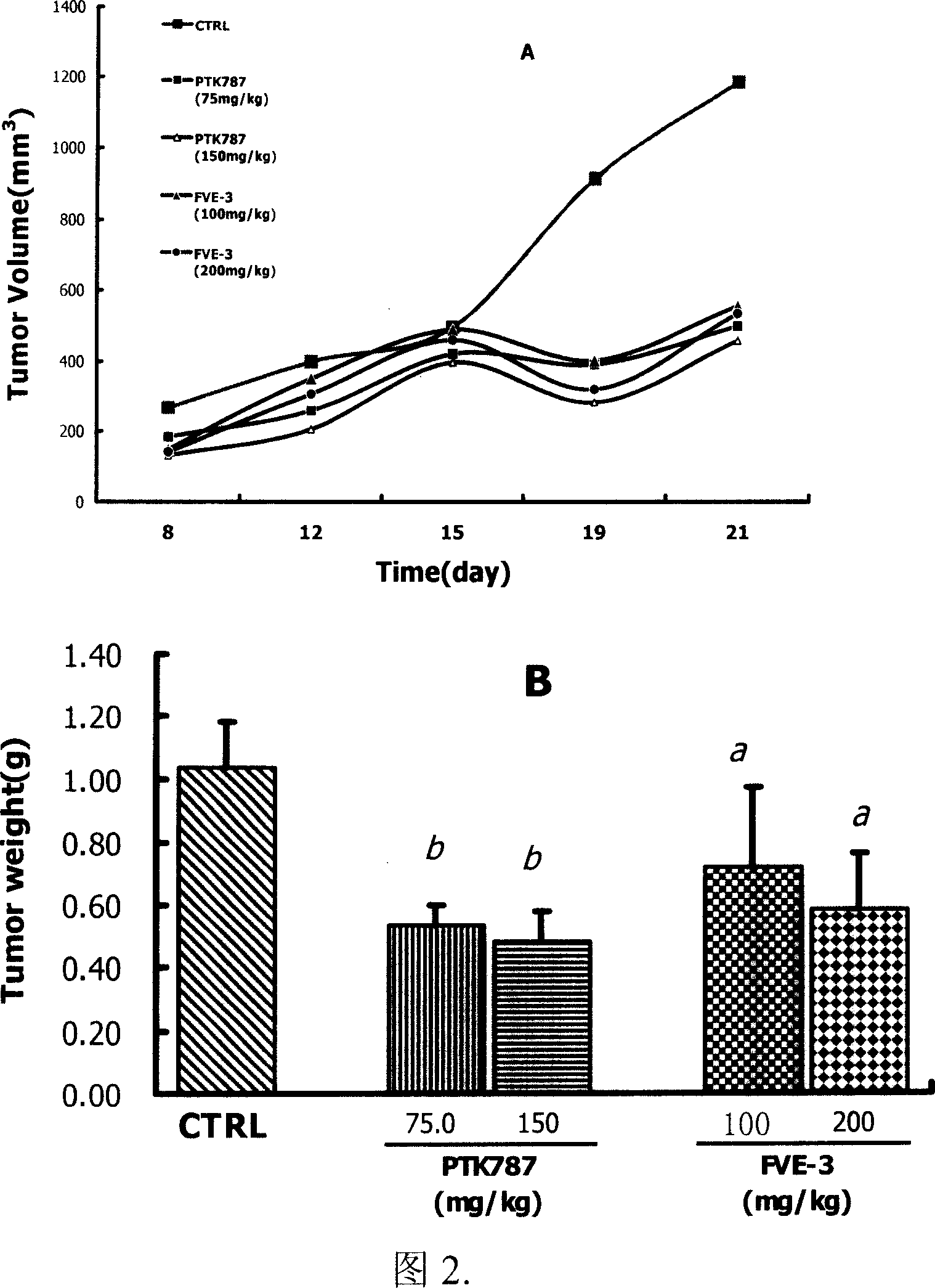
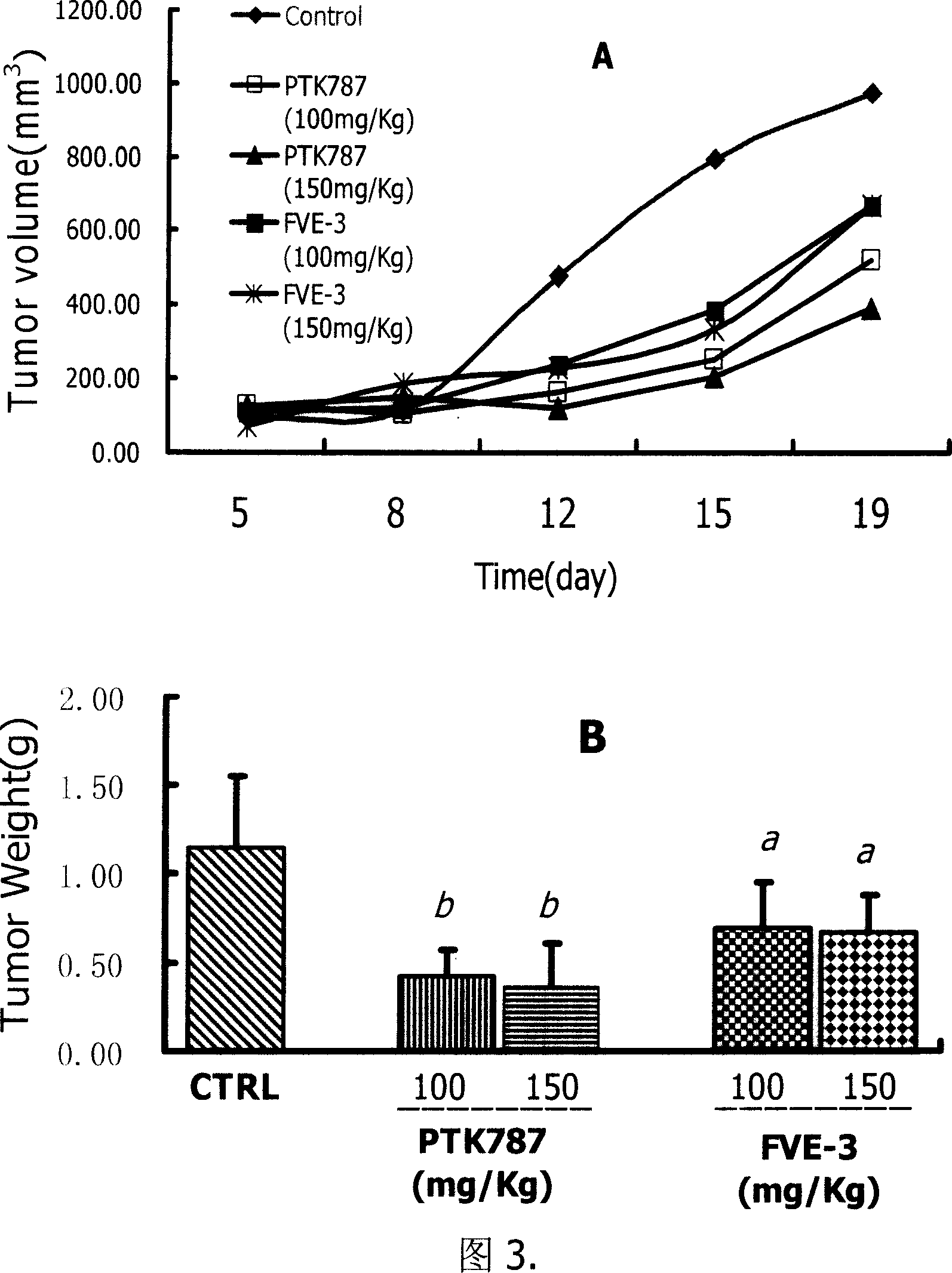
Image
Examples
Embodiment 1
[0048] Example 1 (FVE-3)
[0049]
[0050] 0.48 grams of sodium hydride (60%) (12 mmol) and 50 milliliters of anhydrous tetrahydrofuran were placed in a 100 milliliter flask, and a solution of 1.28 grams (10 millimoles) of p-chloroaniline dissolved in 10 milliliters of anhydrous tetrahydrofuran was added dropwise with stirring , then heated to reflux for one hour, cooled to room temperature, added 2.6 grams (12 mmol) of di-tert-butoxyformic anhydride, stirred for 0.5 hours and then heated to reflux for 14 hours, cooled to room temperature, neutralized by adding saturated ammonium chloride aqueous solution, and Wash with saturated sodium bicarbonate, water, and saturated saline solution, dry over anhydrous sodium sulfate, and spin off the solvent to obtain N-tert-butoxyformyl-p-chloroaniline, which is directly used in the next reaction after drying.
[0051] 1 H NMR (400MHz, CDCl 3 ), δ (ppm) 7.32-7.22 (m, 4H, ArH), 6.48 (S, 1H, NH), 1.53 (S, 9H, CH3).
[0052] MS-FAB: M ...
Embodiment 2
[0058]
[0059] Dissolve 346 mg (1 mmol) of 1-(4-chloroanilino)-4-(4-pyridylmethylene)phthalazine in 15 mL of dry N,N-dimethylformamide, add 1 mL of anhydrous Triethylamine, then dropwise added 129 mg (1.2 mmol) of ethoxyformyl chloride and 5 ml of N,N-dimethylformamide solution, stirred at room temperature until the raw material point disappeared, poured into 100 ml of ice water, acetic acid Extracted three times with ethyl ester, combined the extracts, washed with water and saturated aqueous sodium bicarbonate solution, dried over anhydrous sodium sulfate, spun off the solvent, and separated the residue by column chromatography to obtain the title product.
[0060] 1 H NMR (400MHz, CDCl 3 ), δ (ppm) 8.88 (d, 1H, J=8.0Hz, ArH), 8.00 (m, 2H, ArH), 7.85 (m, 1H, ArH), 7.60 (m, 5H, ArH), 7.02 (m , 1H, ArH), 6.78(m, 2H, ArH), 6.37(d, 1H, CH2), 5.88(S, 1H, CH2), 4.42(m, 2H, CH2), 1.41(t, 3H, CH3) .
[0061] MS-FAB: M + +1 = 419.8.
Embodiment 3
[0063]
[0064] Dissolve 346 mg (1 mmol) of 1-(4-chloroanilino)-4-(4-pyridylmethylene)phthalazine in 10 mL of dry N,N-dimethylformamide, add 1 mL of anhydrous Triethylamine, then dropwise add 163 milligrams (1.2mmol) isobutoxyformyl chloride and 5 milliliters of N, the solution that N-dimethylformamide forms, stir at room temperature until raw material point disappears, pour into 100 milliliters of ice waters, Extract with ethyl acetate three times, combine the extracts, wash with water and saturated aqueous sodium bicarbonate solution, dry over anhydrous sodium sulfate, spin off the solvent, and separate the residue by column chromatography to obtain the title product.
[0065] 1 H NMR (400MHz, CDCl 3 ), δ (ppm) 8.68 (d, 1H, ArH), 7.89 (m, 3H, ArH), 7.59 (m, 1H, ArH), 7.43 (m, 2H, ArH), 7.22 (m, 3H, ArH) , 6.90(m, 2H, ArH), 6.25(d, 1H, CH2), 5.98(S, 1H, CH2), 4.11(m, 2H, CH2), 2.05(m, 1H, CH), 0.99(d, 6H, CH3).
[0066] MS-FAB: M + +1 = 447.9.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com