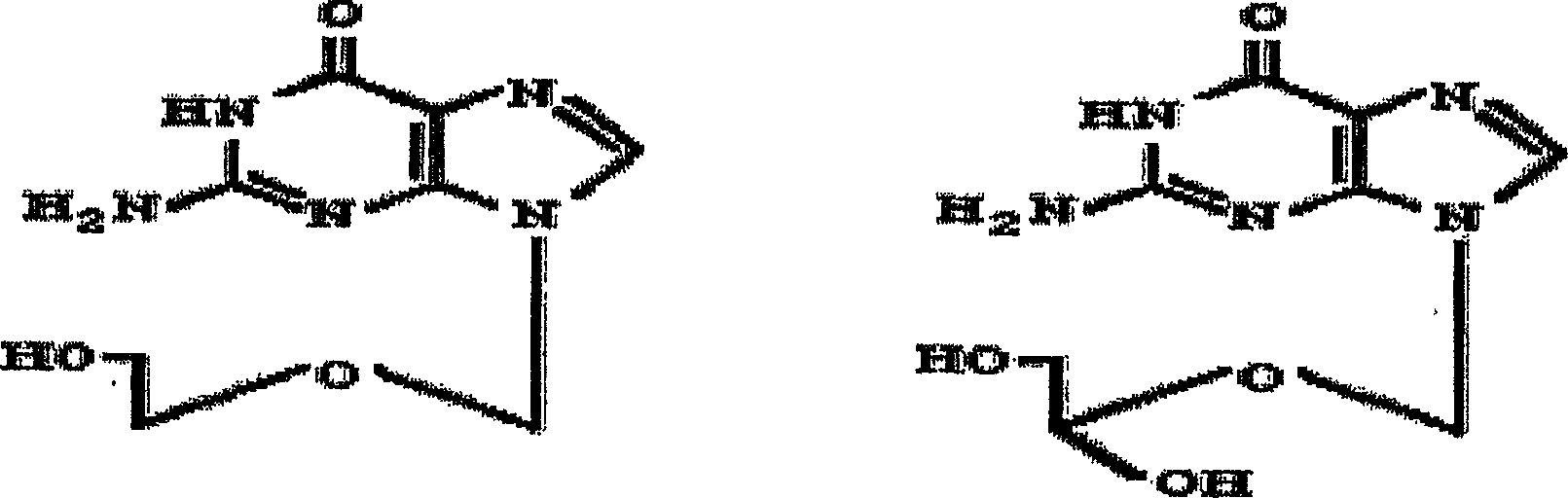Ready-for-use injectable solution of 9-((1,3-dihydroxypropan-2-iloxy)methyl)-2-amine-1h-purin-6(9h)-one
A technology of dihydroxy and oxy groups, applied in the field of injection solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0056] Preparation of 9-((1,3-dihydroxypropan-2-yloxy)methyl)-2-amino in its basic form -1H-purin-6(9H)-one free acid form without free basic residue method
[0057] Add 15 g of caustic soda to a suspension of 100 g of 9-((1,3-dihydroxypropan-2-yloxy)methyl)-2-amino-1H-purin-6(9H)-one in 1 L of demineralized water, pH 11.5, all dissolved. Thereafter, the temperature of the solution was raised to 85° C., and about 6 g of fuming hydrochloric acid were added until pH=4.5. The solution was cooled to 5 °C and 9-((1,3-dihydroxypropan-2-yloxy)methyl)-2-amino-1H-purin-6(9H)-one (free acid form) crystallized . After stirring at 5°C for 30 minutes, the solid was filtered and washed with isopropanol. Suspend the solid of 9-((1,3-dihydroxypropan-2-yloxy)methyl)-2-amino-1H-purin-6(9H)-one in isopropanol and reflux vigorously for 4 h . The suspension was cooled to room temperature (25°C) and immediately filtered. The resulting 9-((1,3-dihydroxyprop-2-yloxy)methyl)-2-amino-1H-purin...
Embodiment II
[0059] Preparation of 9-((1,3-dihydroxypropan-2-yloxy)methyl)-2-amino in its basic form -1H-purin-6(9H)-one free acid form without free basic residue method
[0060] In a glass reactor equipped with a reflux condenser, 9-((1,3-dihydroxypropan-2-yloxy)methyl)-2-amino-1H-purine-6(9H )-ketone ratio of 10 parts, 9-((1,3-dihydroxyprop-2-yloxy)methyl)-2-amino-1H-purin-6(9H)-one was suspended in demineralized water, Stir vigorously at room temperature until completely homogeneous. Into the resulting suspension under stirring, with respect to 9-((1,3-dihydroxypropan-2-yloxy)methyl)-2-amino-1H-purin-6(9H)-one The equivalent of 1.1 moles of sodium hydroxide is added to sodium hydroxide (caustic soda), and the suspension is completely dissolved. Thereafter, with stirring, the temperature of the solution was raised to 85° C., and fuming hydrochloric acid was added until the pH of the solution was 4.5, using about 5.4 to 6.6 g of hydrochloric acid. In order to crystallize 9-((1,3-di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com