Long acting injectable crystal formulations of estradiol metabolites and methods of using same
A technology of estradiol and metabolites, applied in the field of sustained release formulations of estradiol metabolites, which can solve the problems of short half-life, low oral bioavailability, and sensitivity to chemical degradation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1. Preparation of Estradiol Metabolite Crystals
[0065] A 4% w / v solution of 2ME was prepared in a solvent consisting of 9.2% tetrahydrofuran, 61% methanol, and 28% 6M hydrochloric acid. This solution was added dropwise to an equal volume of vigorously stirred water. The resulting solid was isolated by suction filtration, washed with water and dried under vacuum. The resulting 2ME crystals are a mixture of large hollow prisms greater than 500 μm in length and up to about 200 μm in width, and cubic particles falling from about 50 μm down to about 500 nm square. The broad particle size range is used to generate a complex, biphasic pharmacokinetic profile upon injection.
Embodiment 2
[0066] Example 2. In vivo pharmacokinetics of crystal formulations of estradiol metabolites
[0067] The material produced in Example 1 was ground in a mortar and pestle, sieved through a 180 μm sieve, and the particle size distribution of the sieved material was measured on a Coulter LS13320 particle size analyzer. The measured volume average particle diameter is 48.98±36.95 μm.
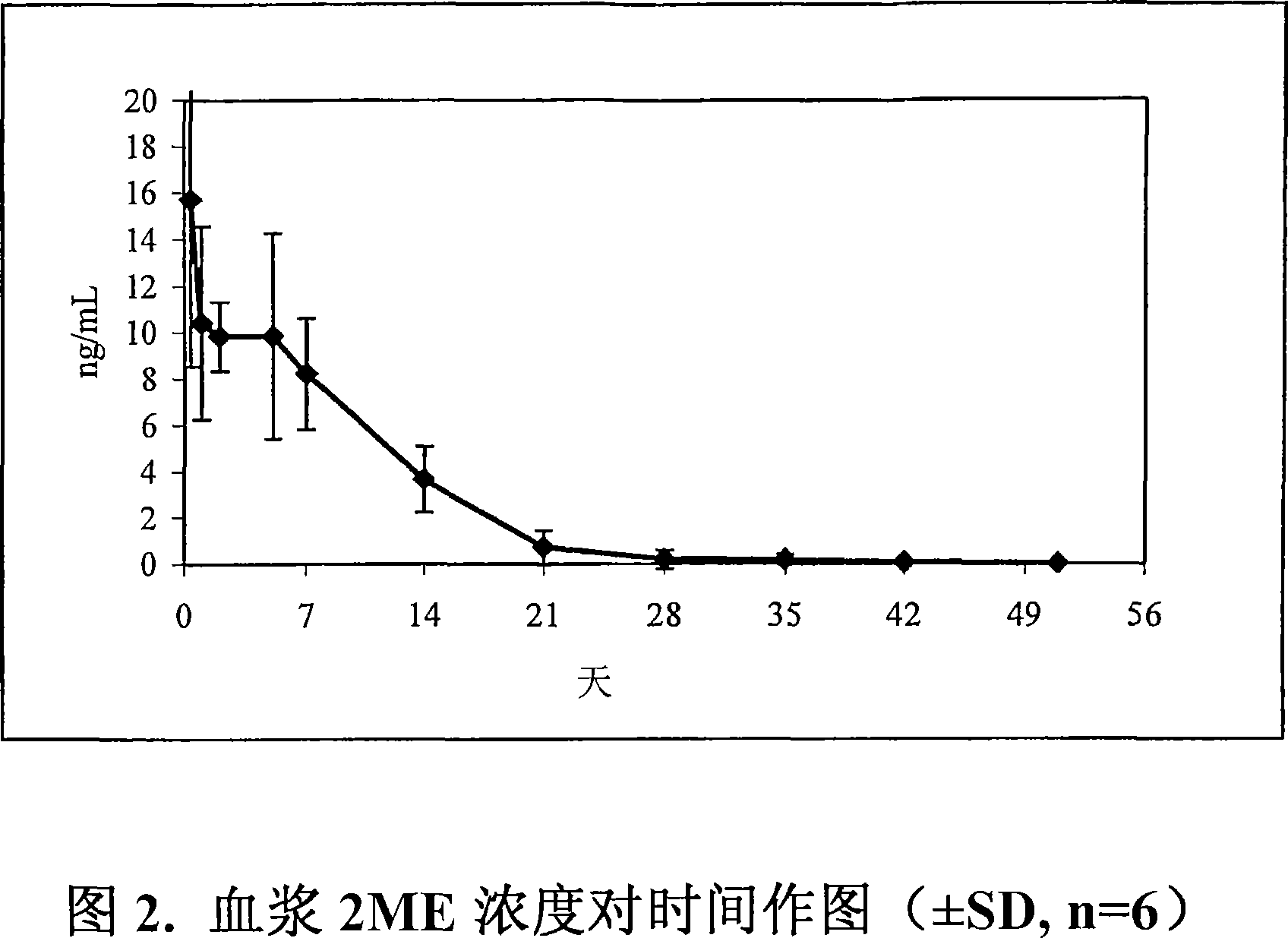
[0068] On day 0, animals in all treatment groups were administered subcutaneously with 5 mg / rat of 0.25 ml of Tween 80 and 0.1% w / v Tween 80 and 0.1% w / v using a 1cc syringe and a 1″ 20-gauge needle. 2ME in an injection vehicle consisting of 2.0% w / v sodium carboxymethylcellulose of SDS. On day 3, blood was collected from all rats via the tail vein. On days 1, 3, 7, 21, 28, And 35 days, make all rats bleed through lateral tail vein.Extract plasma sample, derivatization, and utilize qualified gas chromatography-mass detection method to measure 2ME concentration.Pharmacokinetic characteristics are as...
Embodiment 3
[0069] Example 3. Esterification of Estradiol Metabolites to Change Water Solubility
[0070]2-Methoxyestradiol is esterified to form 3-benzoyl-2-methoxyestradiol. The water solubility of 2ME is about 0.002 mg / ml at room temperature. The water solubility of the esterified compound is about 3 times lower under the same conditions. 2-Hydroxyestradiol is esterified to form 3-hydroxyestradiol-1,3,5(10)-triene-2,17β-diol diacetate. The water solubility of 2-hydroxyestradiol is about 0.155 mg / ml at room temperature. The water solubility of the esterified compound is about 25 times lower under the same conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com