Method of separating and purifying o/p-dihydroxy benzene prepared by phenol hydroxylation
A technology for hydroxylation of phenol and hydroquinone, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of increasing energy consumption for de-catechol, equipment manufacturing, and maintenance costs, and achieves The effect of energy consumption reduction, carbonization prevention, and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
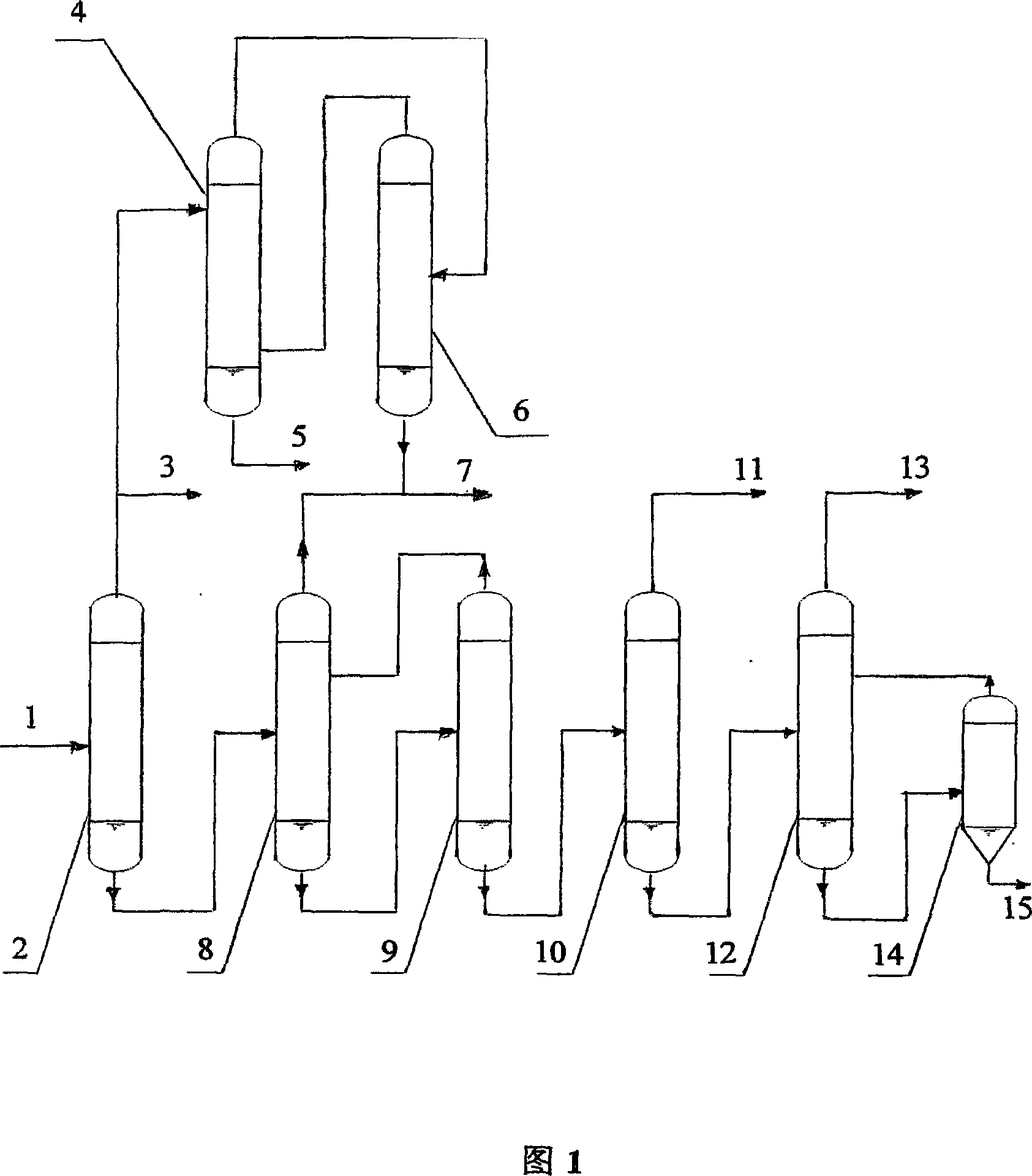
[0024] The raw material liquid is sent into the dehydration tower 2 at 211.9kg / h, and the composition of the raw material is shown in Table 1.
[0025]The dehydration tower 2 is operated under normal pressure, the reflux ratio is 0.2, and the temperature of the tower kettle is 185-190°C. An aqueous solution 3 containing 10.4% phenol is obtained at the top of the tower, 38% of which is sent to the extraction tower 4 to recover phenol 7, and the rest is reused in the circulating reaction system. The composition of the output from the tower kettle is shown in Table 2.
[0026] composition
water
phenol
Quinol
tar
Content / %
37.8
52.8
4.5
2.7
2.2
[0027] composition
water
phenol
Catechol
Quinol
tar
Content / %
0.8
83.1
7.7
4.6
3.8
[0028] The 3 fractions of the phenol aqueous solution are sent to the rotary disk extraction c...
Embodiment 2
[0039] The raw material liquid is sent into the dehydration tower 2 at 211.9kg / h, and the composition of the raw material is shown in Table 7.
[0040] composition
water
phenol
Catechol
Quinol
tar
Content / %
57.8
32.8
4.5
2.7
2.2
[0041] composition
water
phenol
Catechol
Quinol
tar
Content / %
0.3
73.6
12.5
7.5
6.1
[0042] The dehydration tower 2 is operated under normal pressure, the reflux ratio is 0.5, and the temperature of the tower kettle is 185-190°C. A 10.0% aqueous solution containing phenol fraction is obtained at the top of the tower, 38% of which is sent to the extraction tower 4 to recover phenol, and the rest is reused in the circulating reaction system. The composition of the output from the tower kettle is shown in Table 8.
[0043] The phenol aqueous solution fraction is sent to the rotary disk extraction column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com