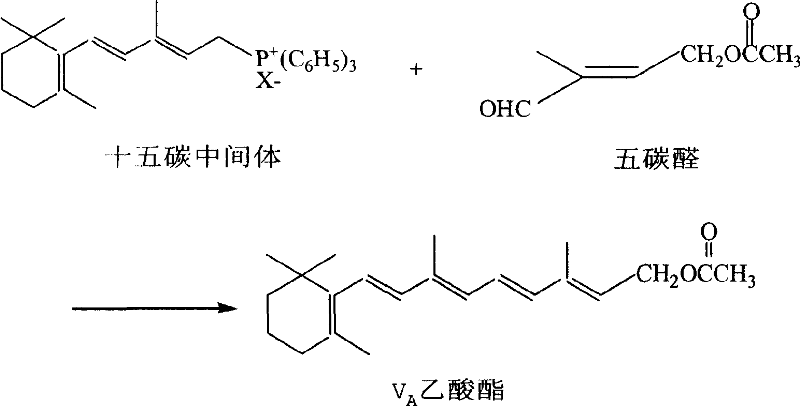
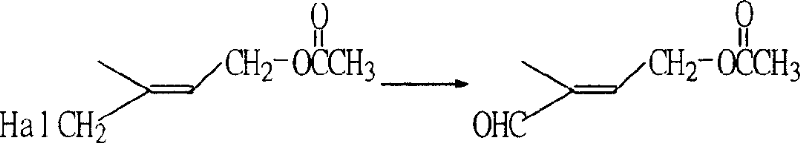
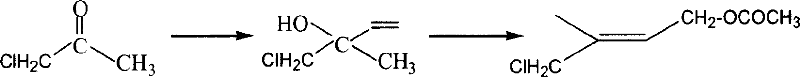Preparation method of 1-chlorin -2-methyl -4-acetoxy-2- butylene
A technology of acetoxy and methyl, which is applied in the chemical field, can solve the problems of increased impurities and poor product content, and achieve the effects of convenient storage and transportation, less miscellaneous ions, and a mild reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of 1-chloro-2-hydroxyl-2-methyl-3-butene and 1-chloro-2-methyl-4-hydroxyl-2-butene mixture
[0023] Put the 1000ml four-neck bottle equipped with a thermometer, a solid feeding port and a dropping funnel into an alcohol cooling bath; add 68g (1mol) isoprene, 100ml water and 0.1g inhibitor hydroquinone; Put 150g of 50% acetic acid aqueous solution in the funnel, stir and add the acetic acid aqueous solution dropwise at 0-5°C, while adding slowly, add bleaching powder (30% available chlorine) in batches from another feeding port, totaling 150g, and continuously measure the pH value , keep the pH value between 7.5 and 8.5 (test paper or pH meter can be used), after about three hours, the dropwise addition is completed, then continue to insulate and stir for 1 hour, filter, wash the filter cake with dichloromethane, and leave the filtrates to separate after merging. After the organic layer was washed with water, 118 g of the crude product was recovere...
Embodiment 2
[0024] Example 2: Preparation of 1-chloro-2-hydroxyl-2-methyl-3-butene and 1-chloro-2-methyl-4-hydroxyl-2-butene mixture
[0025] Ratio of materials, operating temperature and aftertreatment are the same as in Example 1, the difference is that 50% acetic acid aqueous solution is disposablely added in the reaction flask, bleaching powder (30% available chlorine) is added in batches, and the pH value of the system is constantly changing, from the beginning The 3 or so becomes between 7.5 and 8 at the end. 103 g of crude product was obtained, gas phase analysis showed that the total product content was 86.5%, and the yield was 74%.
Embodiment 3
[0026] Example 3: Preparation of 1-chloro-2-methyl-4-acetoxy-2-butene by esterification rearrangement reaction
[0027] In a 250ml three-necked flask, add 64g (content 90.5%, 0.48mol) of the crude product obtained in Example 1 and 80g (0.78mol) of acetic anhydride, stir and add 1g p-toluenesulfonic acid, heat up to 60°C and stir for 5 hours, then cool down. Add 100ml of water to separate the layers, discard the upper waste water, add 100ml of water to the lower organic layer and wash with water to obtain 63g (content 90%) of crude product chloride. %. GC-MS (m / e): 127, 102, 84, 67, 43 (100%), 29; IR (ν / cm -1 ): 1735 (-OCO-, carbonyl); 1230 (-C-O-CO-, ν as )1035(-C-O-CO-,ν s ); 1 HNMR (500MHz, CDCl 3 )δ (ppm): 1.83 (s, 3H, -CH 3 ); 2.06(s,3H,-COCH 3 ); 4.01 (2H, Cl-CH 2 -); 4.62 (2H, =CH 2 ); 5.69 (1H, -CH=); DEPT: δ (ppm): 124.019 (1H, =CH-); 62.535 (2H, -OCH 2 -); 50.135 (2H, -CH 2 -Cl); 21.106 (3H, -CH 3 ); 14.807 (3H, -CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com