Simple method for ozone oxidation preparation of alpha-FeOOH, beta-MnO2 and Co3O4 nano material
A nano-material and ozone oxidation technology, applied in the direction of manganese oxide/manganese hydroxide, cobalt oxide/cobalt hydroxide, iron oxide/iron hydroxide, etc., can solve the problem of less application of ozone, and achieve low cost and various shapes , the effect of simple response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
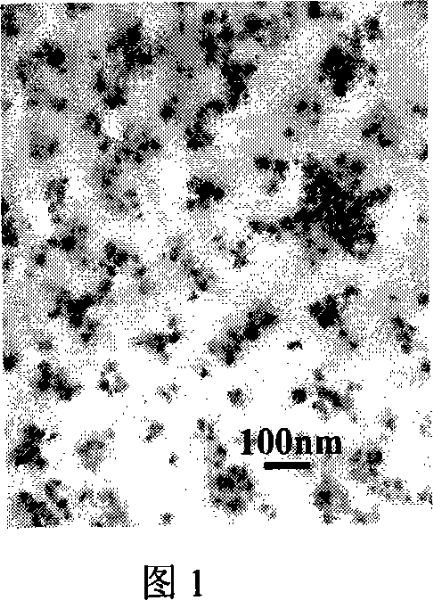
[0039] Weigh FeSO 4 ·7H 2 O 1.20g was dissolved in 40ml water, and ozone was passed through with 0.30mg / min flow for 30 minutes under stirring, and the precipitate was washed with distilled water and dried to obtain FeOOH nanoparticles with a particle diameter of 20-40 nanometers. The electron microscope photo is shown in Figure 1 .
Embodiment 2
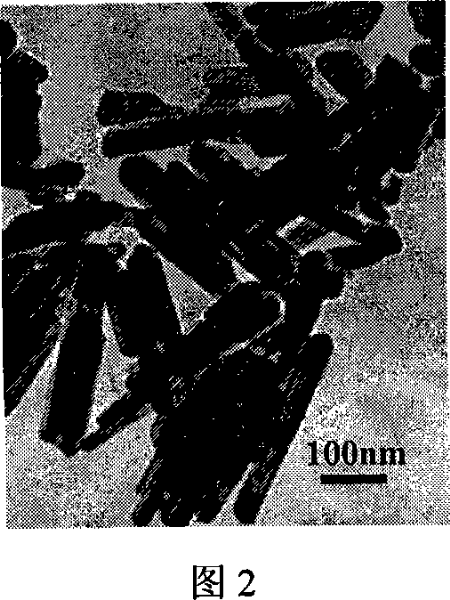
[0041] Weigh FeSO 4 ·7H 2 O 1.20g was dissolved in 40ml of water, and ozone was passed through at a flow rate of 0.30mg / min for 30 minutes under stirring, transferred to a closed reactor and heated at 150°C for 8 hours, after cooling, the precipitate was washed with distilled water and dried to obtain a diameter of 30 nanometers The electron micrographs of FeOOH nanorods with a length of about 200 nanometers are shown in Figure 2.
Embodiment 3
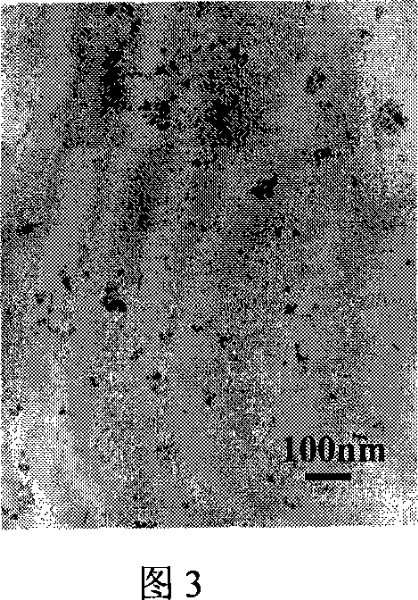
[0043] Weigh Co(CH 3 COO) 2 4H 2 O 0.50g was dissolved in 20ml of water, and ozone was passed through at a flow rate of 0.30mg / min for 30 minutes under stirring, transferred to a closed reactor and heated at 150°C for 4 hours, after cooling, the precipitate was washed with distilled water and dried to obtain a particle size of 10-20 nm Co 3 o 4 nanoparticles. Electron microscope photos are shown in Figure 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com