Toad sterene compounds and application thereof in pharmaceutical preparation
A technology of bufastene and compound, applied in the field of medicinal chemistry and its preparation, can solve the problems of low anti-tumor cell activity, low water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
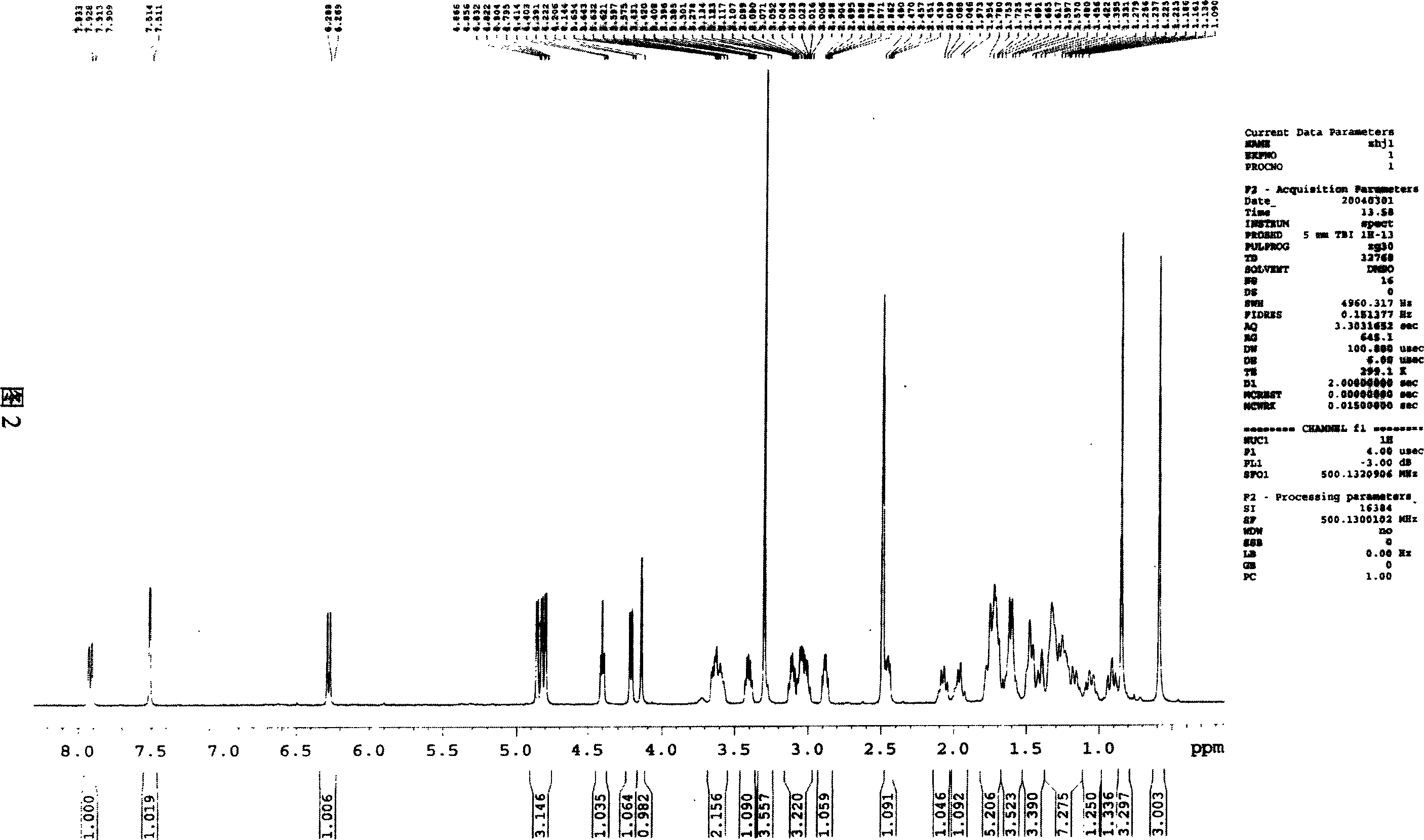
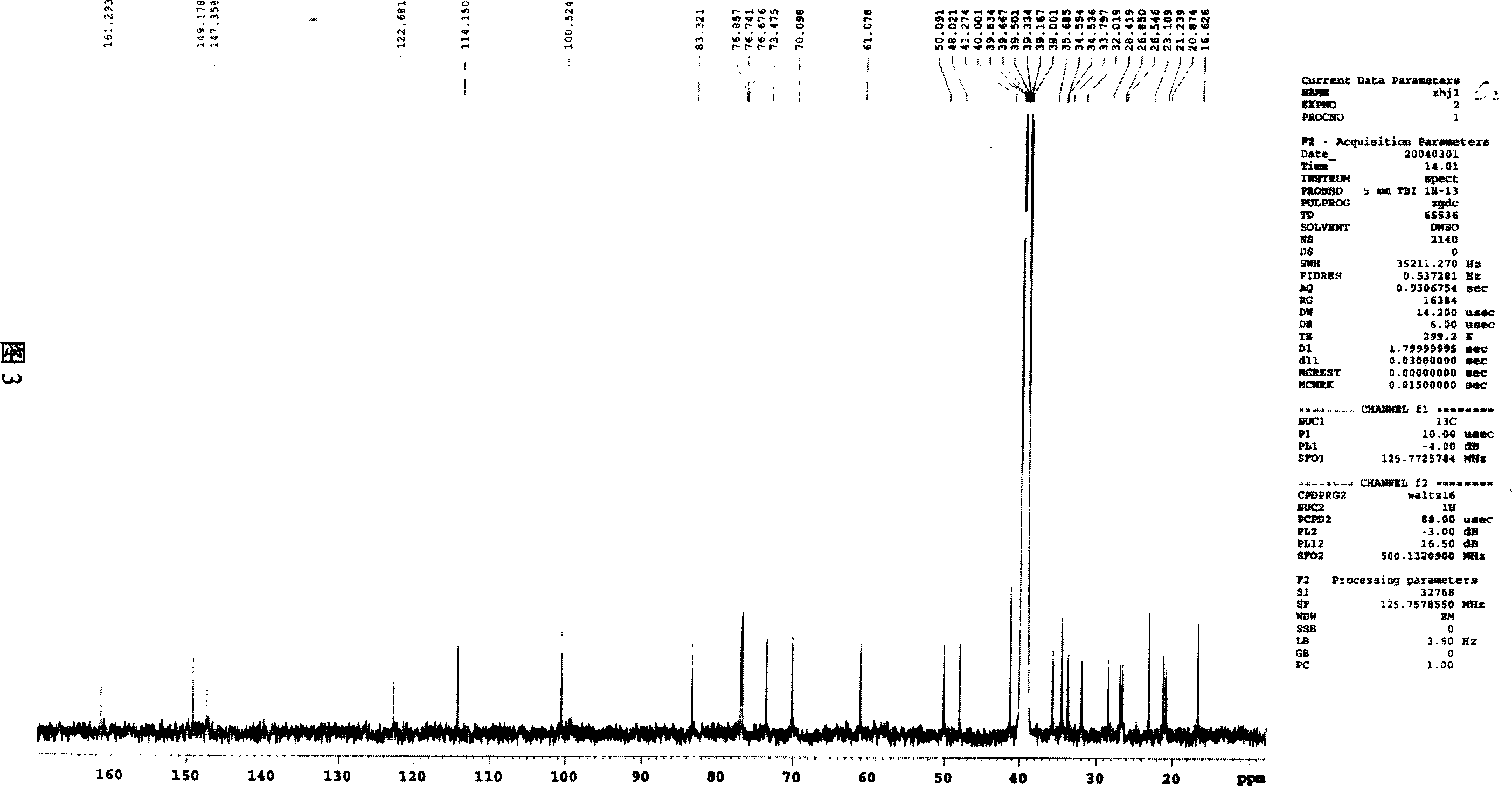
Image
Examples
Embodiment 1
[0060] Extraction and separation of the toad poison substrate: the toad venom extract is dissolved in water to form 10 liters of homogenized samples, extracted with chloroform (10 L x 4 times), the chloroform extract is dissolved in a small amount of chloroform acetone, mixed with 3 times the amount of silica gel, and removed The solvent, after being completely dried, was loaded into a silica gel column. The silica gel column has a diameter of 4.5 cm and a length of 60 cm. The silica gel pH 5.3 has a particle size of 100-200 mesh. For elution, the eluent is acetone-cyclohexane (1:5) eluent with different gradients. Collected in sections, fractions 5-10 were obtained to obtain Cinobufagin, fractions 11-13 to obtain Cinobufagin, fractions 14-15 to obtain Cinobufen essence and bufalin, fractions 16-20 to obtain toad Toad spirit, fraction 33-35 get toad toad spirit. Fractions 36-42 Deyuan Cinobufina Toxin. A small amount of cinobufagin and bufafolin in fractions 14-15 were fur...
Embodiment 2
[0062] Obtaining of bufasterene compounds:
[0063] Pre-experiment: add bufafolin, bufabugenin, cinobufagin, bufabufalin and bufafolin to the suspension culture medium of periwinkle cells, at 25°C for 12 hours Co-cultured under light conditions, extracted with ethyl acetate, concentrated, dissolved in a small amount of acetone. Petroleum ether: Acetone is used as a developing solvent, 10% sulfuric acid alcohol is used for color development at 120° C., and the results are analyzed by TLC (silica gel G, 0.3% CMC-Na binder).
[0064] Transformation product amplification: 15 mg / mL of mixed bufotoxin substrate containing bufalin, bufabugenin, cinobufagin, bufabufalin and bufafetoxin was put into the suspension cell liquid of Changchun flower, and the culture medium It is MS+NAA0.5mg / L+6-BA0.5mg / L+sucrose 3%, pH5.8. Co-cultivate at 25°C for 12 hours under light or dark conditions, extract with ethyl acetate, concentrate, combine and recover.
Embodiment 3
[0068] MTT method was used to measure the cytotoxic activity of 3-epi-bufalin 3-O-β-D-glucoside: HL-60 cell line was cultured in 20% RPMI 1640 medium, and the cell density was adjusted to 0.5×10 5 / mL, planted in a 96-well plate, bufalin and the transformation product were dissolved in methanol, according to the blank, methanol control, bufalin (10mg / L, 1mg / L, 0.5mg / L, 0.1mg / L, 0.05 mg / L), 3-epi-bufalin 3-O-β-D-glucoside (10mg / L, 1mg / L, 0.5mg / L, 0.1mg / L, 0.05mg / L) design concentration added to HL The -60 cell line was cultured in a CO2 incubator for 44 hours and MTT was added, and SDS was added for 48 hours to terminate the reaction.
[0069] Bufalin and 3-table-bufalin 3-O-β-D-glucoside inhibit the leukemia HL-60 cell line results are shown in Table 1, and the analysis of variance in Table 2 shows that 3-table-bufalin Compared with bufalin, 3-O-β-D-glucoside has significantly improved cytostatic activity.
[0070] Table 1 The colorimetric results of the inhibition of bufali...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com