Quality-control method of a traditional Chinese medicine 'Xuebijing' injection
A quality control method and Xuebijing technology, applied in the field of removing blood stasis and detoxification, can solve problems such as difficulty in effectively controlling the quality of preparations, affecting product production and ensuring quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Preparation of Xuebijing Injection:
[0081] The formula consists of the following:
[0082] 100g of safflower, 100g of red peony root, 100g of Chuanxiong, 100g of salvia miltiorrhiza, 100g of angelica,
[0083] Its preparation method is as follows:
[0084] [Preparation method] the above five flavors, take safflower, according to the percolation method (Appendix 10 of Chinese Pharmacopoeia 2005 edition) under the liquid extract and extract item, use 8 times the amount of 30% ethanol as solvent, soak for 8 hours, soak Filter, collect 5 times the amount of filtrate, add ethanol to process twice, the first time to make the ethanol content reach 70%, the second time to 80%, each time refrigerated at 5 ° C for 48 hours, filter, the filtrate recovers ethanol, concentrates to 5 times the amount, vacuum-dry it into a dry paste, dissolve it with 0.5 times the amount of water for injection, and set aside; add water to decoct the Radix Paeoniae Rubra twice, add 10 times the amo...
Embodiment 2
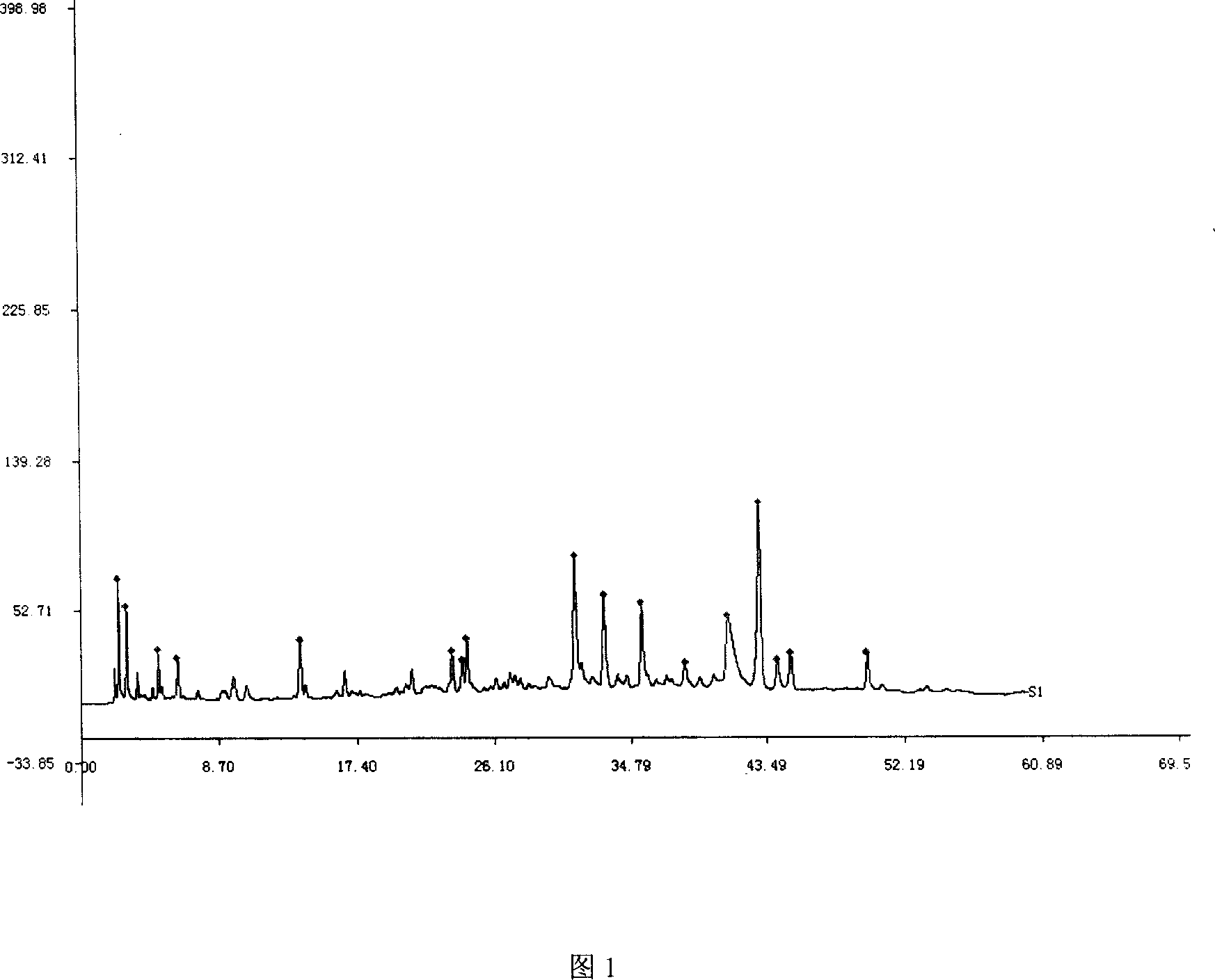
[0086] The steps to compare the fingerprints of Xuebijing Injection are:
[0087] [Fingerprint] With reference to high-performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2005 Edition), combined with the requirements of fingerprints, it was determined. Proceed as follows:
[0088] 1. Formulate standard control fingerprints;
[0089] 2. Measure the fingerprint of the preparation of the present invention;
[0090] 3. Compare the above fingerprints;
[0091] specifically is:
[0092] Obtain the high performance liquid chromatogram of standard product paeoniflorin with high-performance liquid chromatography, the preparation of standard product paeoniflorin solution Take paeoniflorin (standard reference substance can be purchased in the drug supervision department) reference substance amount, accurately weighed, add methanol to make A solution containing 1.0 mg per 1 ml is available.
[0093] The specific chromatographic conditions are as follows:
[0094]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com