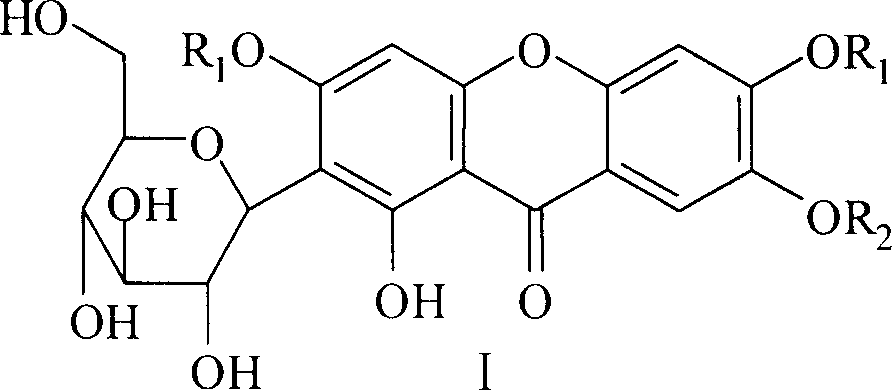
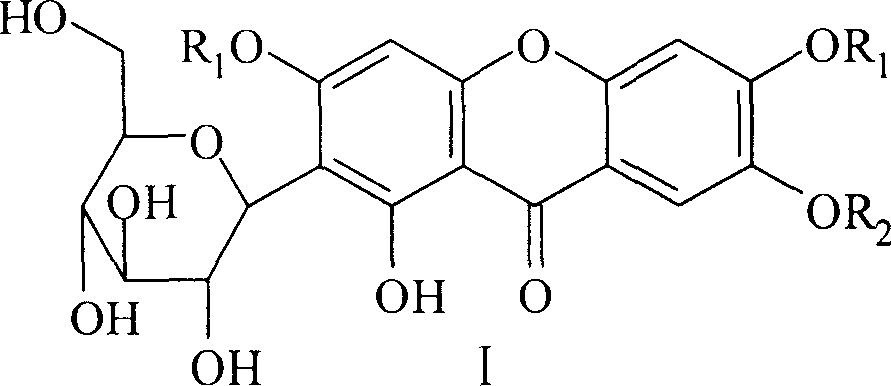
Glycoside class compound of mango, preparation method, and application in area of medicine
A compound, the technology of mangiferin, which is applied in the field of mangiferin compounds, can solve the problems of poor solubility and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Prepare the compound according to the above method, light yellow crystal; yield: 56.3%; melting point: 170~172°C; IR(KBr)cm 1 : 3430, 2972, 2934, 2879, 1648, 1606, 1468, 1381, 1278, 1198, 1090, 1028, 905, 811, 791, 657, 578; 1 HNMR (DMSO-d 6, δ): 13.50(1H, s), 7.50(1H, s), 7.16(1H, s), 6.61(1H, s), 4.83(2H, b), 4.63(1H, m), 4.60~4.55( 2H, m), 4.54 (1H, b), 4.36 (1H, b), 4.28 (1H, m), 4.04 (1H, m), 3.75~3.66 (2H, m), 3.18 (2H, m), 3.05 (1H, m), 1.77-1.61 (6H, m), 1.34-1.24 (9H, m), 1.05-0.93 (9H, m).
Embodiment 2
[0027] Example 2: Synthesis of 2-C-β-D-glucopyranosyl-3,6,7-tri(1-heptyl)-1-hydroxyl-xanthone (compound 6 in Table 1)
[0028] Prepare the compound according to the above method, light yellow crystal; yield: 66.2%; melting point: 122~123°C; IR(KBr)cm -1 : 3438, 2928, 2856, 1648, 1609, 1580, 1507, 1466, 1394, 1277, 1226, 1200, 1111, 1085, 1030, 901, 823, 807, 724, 661, 632, 576; 1 HNMR (DMSO-d 6 , δ): 13.48(1H, s), 7.46(1H, s), 7.07(1H, s), 6.55(1H, s), 4.69(2H, b), 4.63(1H, m), 4.31(1H, b), 4.15(1H,b), 4.07(6H,m), 4.05(1H,m), 3.74(1H,m), 3.45(1H,m), 3.23(2H,m), 3.15(1H,m ), 1.78 (6H, m), 1.47 (6H, m), 1.31 (18H, m), 0.89 (9H, m).
Embodiment 3
[0029] Example 3: Synthesis of 2-C-β-D-glucopyranosyl-3,6,7-tribenzyl-1-hydroxyl-xanthone (compound 11 in Table 1)
[0030] Prepare the compound according to the above method, light yellow crystal; yield: 54.4%; melting point: 133~134°C; IR(KBr)cm 1 : 3413, 3062, 3031, 2874, 1646, 1609, 1581, 1500, 1475, 1391, 1278, 1223, 1188, 1082, 1025, 905, 809, 737, 696, 668, 642, 615, 571, 466; 1 HNMR (DMSO-d 6 , δ): 13.48(1H, s), 7.63~7.29(17H, m), 6.75(1H, s), 5.29(2H, s), 5.22~5.14(4H, m), 4.70(1H, dd), 4.00(1H,t), 3.67(1H,m), 3.45(1H,m), 3.41(4H,b), 3.22(2H,m), 3.10(1H,m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com