Improved method for producing prohexadione
An acid binding agent, diethyl maleate technology, applied in the preparation of carboxylate, organic chemistry and other directions, can solve the problems of high preparation cost and difficult post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
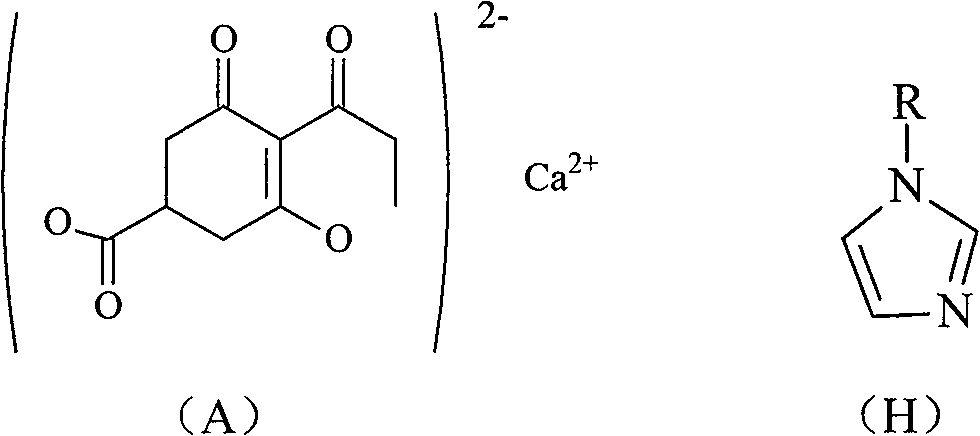
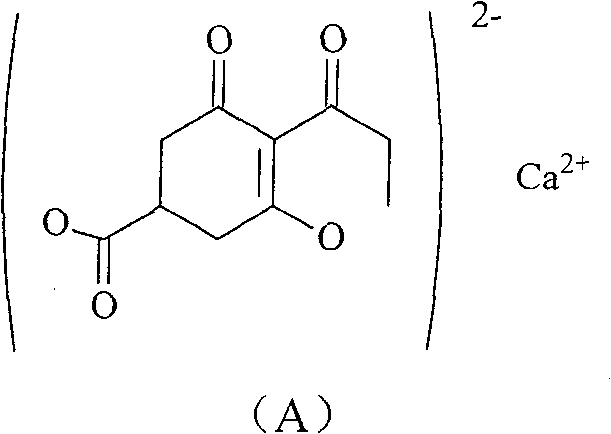
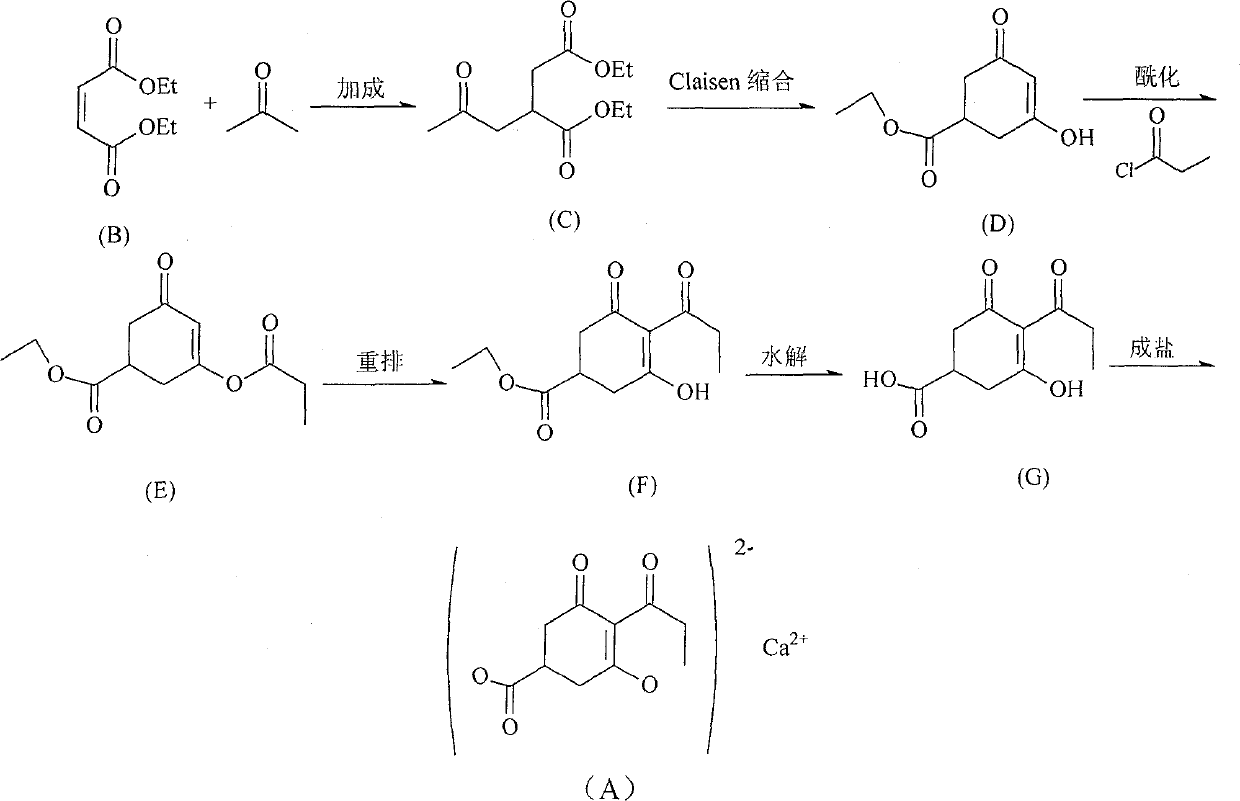
[0015] The method for preparing compound (A) of the present invention comprises the following steps:
[0016] (1) compound (B) and acetone carry out addition reaction to obtain compound (C);
[0017] (2) Compound (C) is condensed by Claisen to obtain compound (D);
[0018] (3) compound (D) reacts with propionyl chloride to obtain compound (E);
[0019] (4) compound (E) is rearranged to obtain compound (F);
[0020] (5) Compound (F) is hydrolyzed to obtain compound (G);
[0021] (6) Compound (G) is salt-formed to obtain the target product [compound (A)].
[0022] Wherein: the reaction conditions of steps (1), (2) and (5) are similar to those of the prior art, please refer to EP 123001 for specific process conditions, and will not repeat them here.
[0023] Step (3) (acylation reaction): prior art (Robert.G.S.Brown, Li Yan, Michael.H.Beale, PeterHedden[J].Phytochemistry, 1998,679-687) adopts dichloromethane as reaction solvent, with 3 times the amount of triethylamine is re...
Embodiment
[0029] (1) Synthesis of 2-acetonyl diethyl succinate [compound (C)]:
[0030] The reaction formula is as follows:
[0031]
[0032] In diethyl maleate (34.4g, 0.2mol), acetone (95.3g, 1.64mol) and diethylamine (2.9g, 0.04mol) drop into 500ml autoclave, be heated to 150 ℃, and the pressure in the still is 1MPa, Insulated for 21 hours, cooled to room temperature, removed low boilers at normal pressure and then distilled under reduced pressure to collect fractions at 155°C-160°C (66Pa) to obtain 39.8g of light yellow liquid with a yield of 86.5%.
[0033] (2) Preparation of 3-hydroxy-5-ethoxycarbonylcyclohex-2-en-1-one [compound (D)]
[0034] The reaction formula is as follows:
[0035]
[0036] 40ml of absolute ethanol and sodium ethoxide (11ml, 0.056mol) were put into the reaction flask, and diethyl 2-acetonylbutyrate (12g, 0.052mol) was added dropwise. After the dropwise addition, it was heated to reflux for 4h. The reaction solution was cooled, concentrated and recla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com