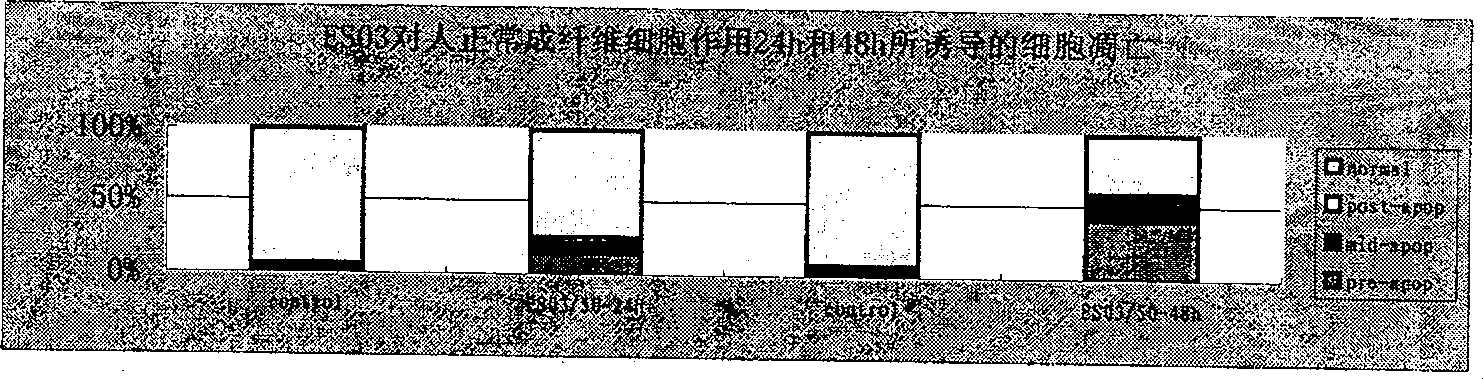
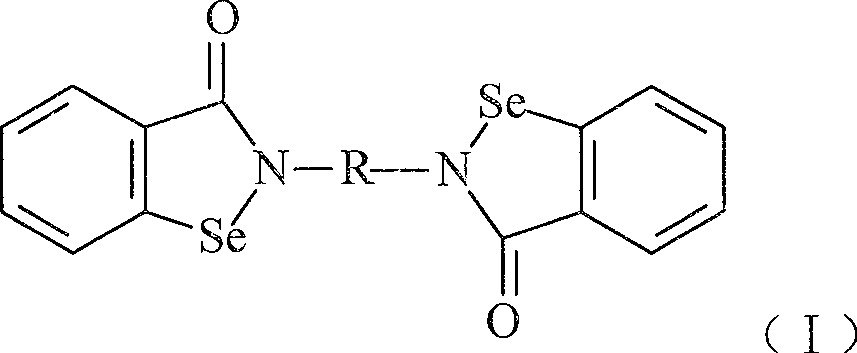
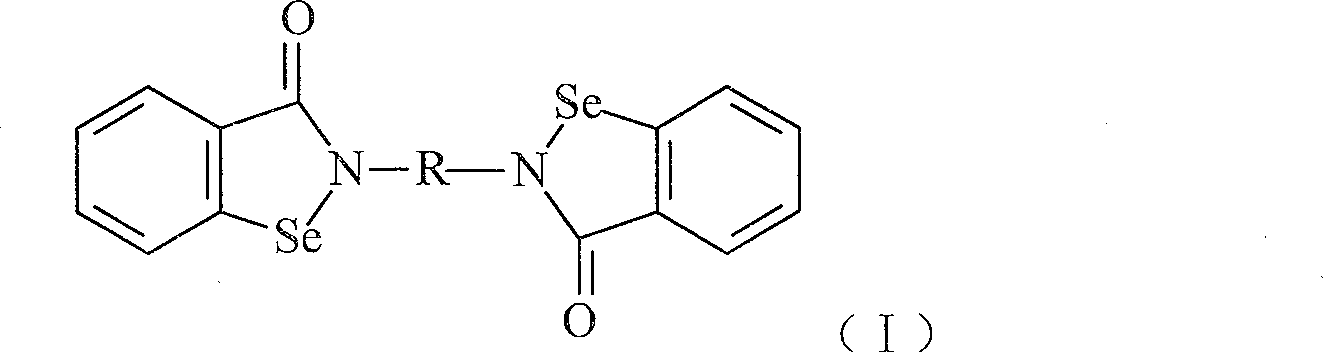
Compound with functions of anti-fibrosis and inhibition of gelatingase activity and use thereof
A compound and gelatinase technology, applied to the active ingredients of heterocyclic compounds, digestive system, drug combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0028] [Example 1] 1, the preparation of 2-[two (1,2-benzisoselenazol-3 (2H)-ketone)] ethane
[0029] 1. Preparation of 2-benzoic acid diazonium chloride
[0030] Under ice bath conditions, accurately weigh 14.0g of anthranilic acid, mix it with 40ml of hydrochloric acid with a concentration of 1:1 under ice bath conditions, then slowly drop 9.0g of sodium nitrite into 20ml The water solution was continued to react for 2 hours after dripping to obtain 2-benzoic acid diazonium chloride for later use.
[0031] 2. Preparation of sodium diselenide
[0032] Add 8.8 g of selenium powder and 4.4 g of sodium hydroxide into 60 ml of deionized water, slowly add 8.8 g of sodium thiosulfate under stirring conditions, and continue to react for 2-4 hours to obtain a sodium diselenide solution for later use.
[0033] 3. Preparation of 2,2'-diselenide bisbenzoic acid
[0034] Under the condition of stirring, the chlorinated 2-benzoic acid diazonium salt solution obtained in step 1 was adde...
Embodiment 2
[0040] [Example 2] 1, the preparation of 3-[two (1,2-benzisoselenazol-3 (2H)-ketone)] propane
[0041] Under the conditions of ice bath and nitrogen protection, 0.82ml 1,3-propanediamine was dissolved in 4.5ml of THF, then 6.45ml triethylamine was added, and 5g of 2-selenochlorobenzoyl chloride was slowly added dropwise to dissolve in In the solution of 60ml THHF, it can be seen that a light yellow solid is continuously formed, and after the dropwise addition, it is raised to room temperature and continues to react for 3 hours. After the reaction, the pale yellow precipitate was filtered out, washed several times with tetrahydrofuran, then washed several times with a large amount of water, and finally washed several times with ethanol and ether, the solid was recrystallized with DMSO-water, and dried to obtain 2.52 g of the product. Yield 58.6%, m.p. 254-257°C. 1 H-NMR (300MHz, DMSO-d 6 )7.39-8.05 (m, 4H, ph-H), 3.78 (t, 2H, CH 2 ), 2.00 (dd, J 1 =6.6,J 2 =6.9,2H,CH 2 ...
Embodiment 3
[0042] [Example 3] 1, the preparation of 4-[bis(1,2-benzisoselenazol-3(2H)-one)] butane (hereinafter referred to as ES-03)
[0043] Under the conditions of ice bath and nitrogen protection, dissolve 0.866ml of 1,4-butanediamine in 50mlTHF, add 5.74ml of triethylamine, and slowly add 4.39g of 2-selenochlorobenzoyl chloride dissolved in 60mlTHF solution, a yellow solid was formed. After the dropwise addition, it was raised to room temperature and continued to react for 3 hours. After the reaction, the pale yellow precipitate was filtered out, washed several times with tetrahydrofuran, then washed several times with a large amount of water, and finally washed several times with ethanol and ether, the solid was recrystallized with DMSO-water, and dried to obtain 2.54 g of the product. Yield 65.7%, m.p.243-248°C
[0044] 1 H-NMR: (300MHz, DMSO-d 6 )δ 7.37-8.04(m, 4H, Hh-H), 3.75(s, 2H, CH 2 ), 1.64 (s, 2H, CH 2 ); elemental analysis C 18 h 16 N 2 o 2 Se 2 Calculated (%):...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com