Slow-released preparation containing hydrochlorothiazide and clonidine hydrochloride and its preparing method
A technology of clonidine hydrochloride and hydrochlorothiazide, which is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulas, etc. It can solve the problems of short duration of drug effects, easy to forget to take and miss doses, and blood drug concentration. Low-level problems, to achieve stable blood drug concentration in the body, reduce the number of administrations, and reduce the effects of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
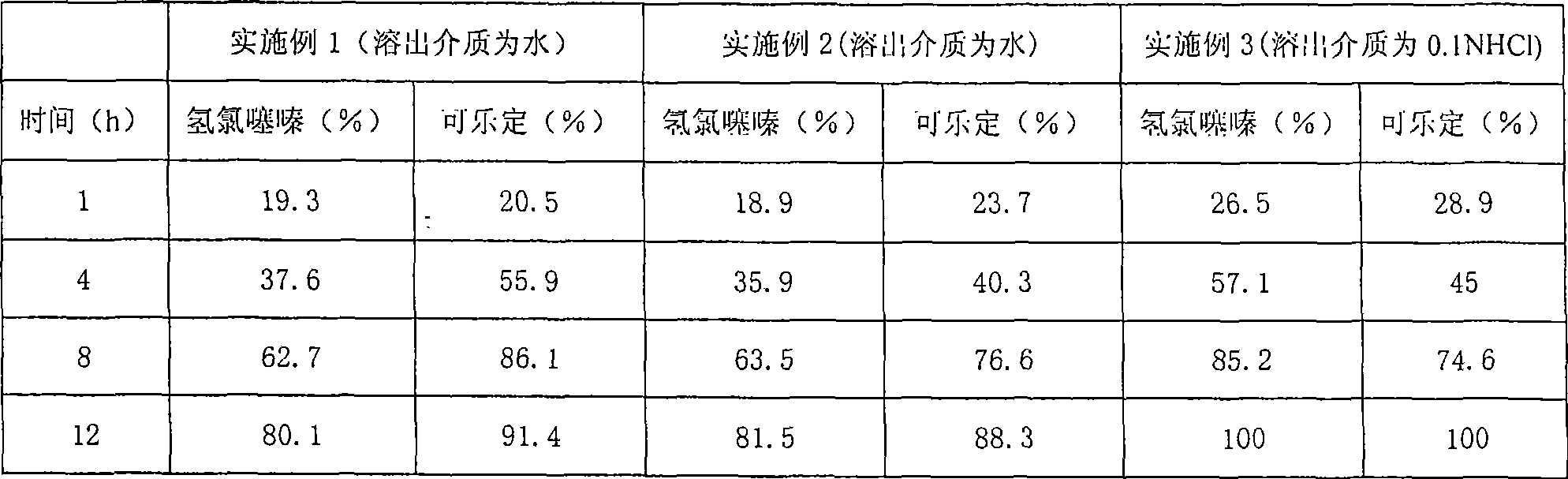
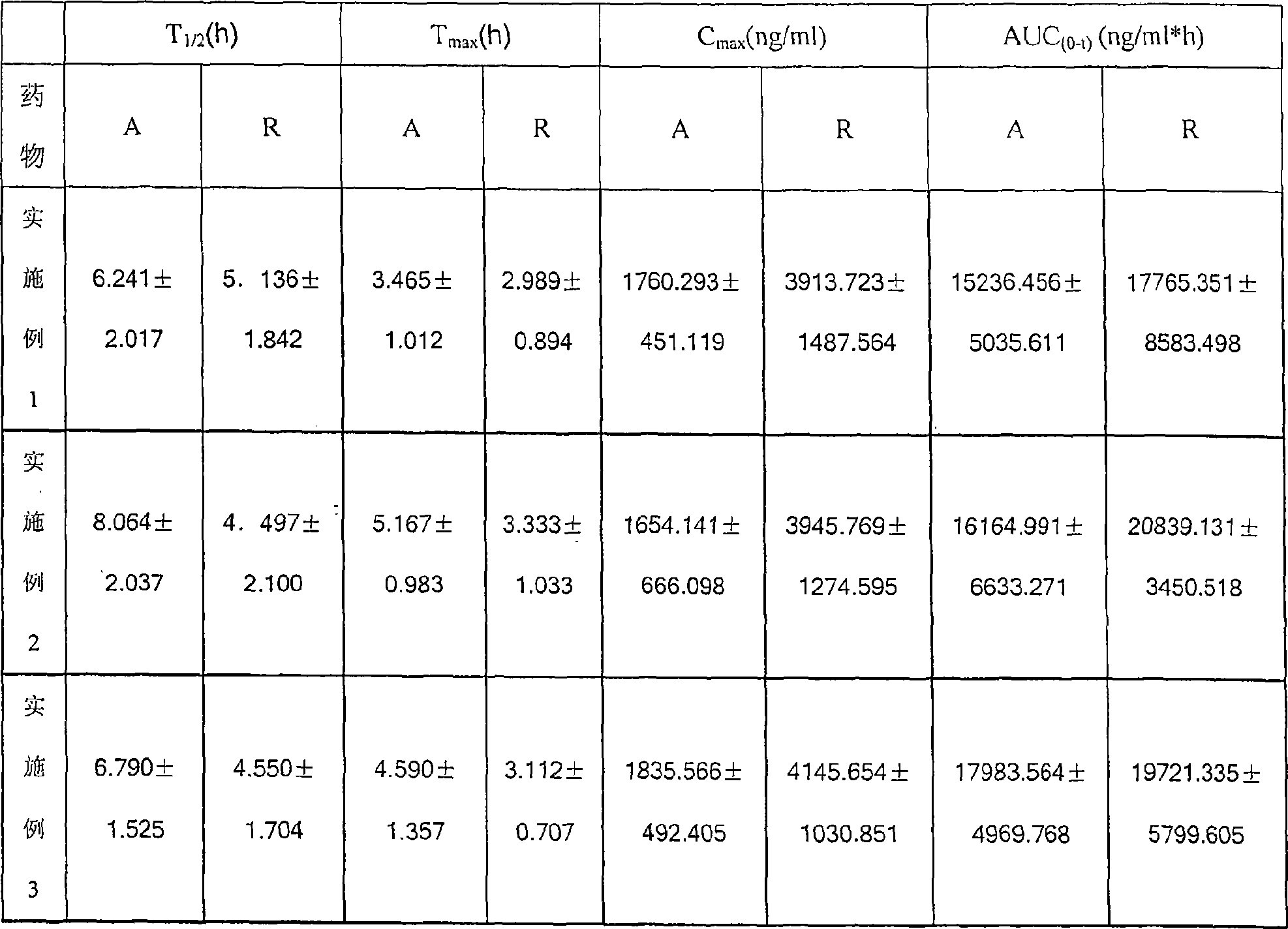
Examples
Embodiment 1
[0033] The following is the amount used in 1000 pieces
[0034] a. Hydrochlorothiazide immediate-release small tablets
[0035] Component Amount (g)
[0036] Hydrochlorothiazide 6
[0037] Microcrystalline Cellulose 10
[0038] Lactose 12.95
[0039] Crospovidone 0.9
[0040] Magnesium stearate 0.15
[0041] b. Hydrochlorothiazide extended-release small tablets
[0042] Component Amount (g)
[0043] Hydrochlorothiazide 31.5
[0044] Hydroxypropyl methylcellulose 12.5
[0045] Kabob 2
[0046] Lactose 8.725
[0047] Magnesium stearate 0.275
[0048] c. Clonidine Hydrochloride Sustained Release Small Tablets
[0049] Component Dosage
[0050] Clonidine hydrochloride 0.1125
[0051] Hydroxypropyl methylcellulose 25
[0052] Carbopol 2.5
[0053] Lactose 22.1375
[0054] Magnesium stearate 0.25
[0055] The preparation process is as follows:
[0056]The raw and auxiliary materials are crushed and sieved, mixed according to the above prescription amount, and then...
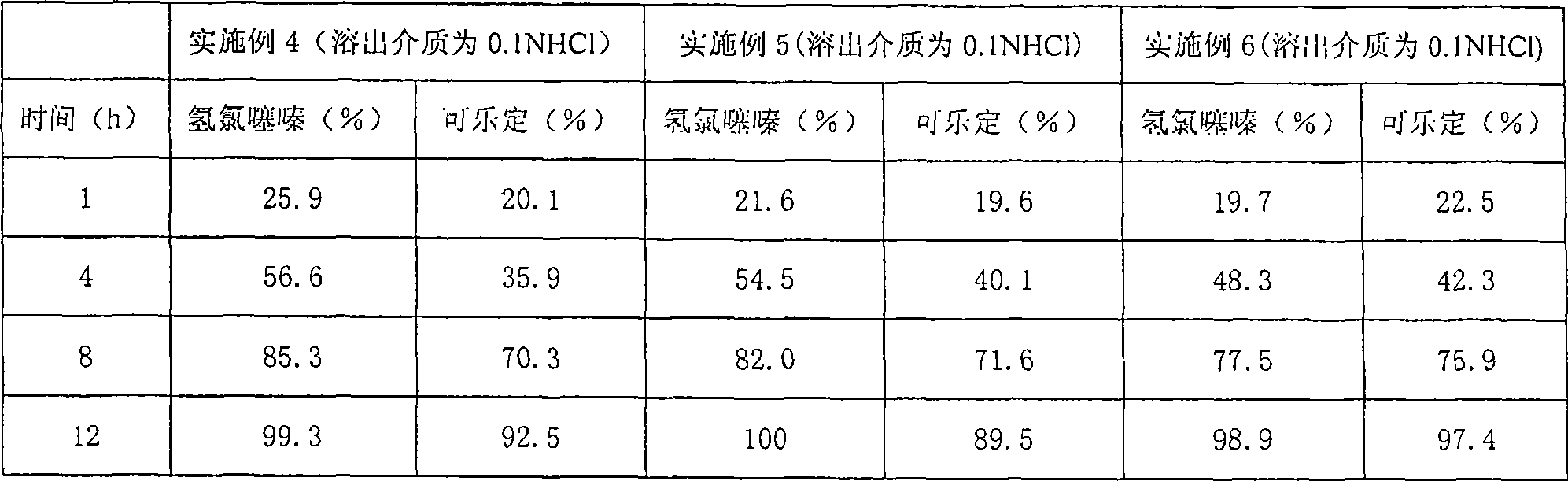
Embodiment 2
[0058] The consumption of present embodiment is the amount used by 1000
[0059] a. Hydrochlorothiazide immediate-release small tablets
[0060] Component Dosage
[0061] Hydrochlorothiazide 6
[0062] Microcrystalline Cellulose 10
[0063] Lactose 12.95
[0064] Crospovidone 0.9
[0065] Magnesium stearate 0.15
[0066] b. Hydrochlorothiazide extended-release small tablets
[0067] Component Amount (g)
[0068] Hydrochlorothiazide 31.5
[0069] Hydroxypropyl methylcellulose K100 10.2
[0070] Kabob 9.6
[0071] Lactose 19.2
[0072] Magnesium stearate 0.3
[0073] c. Clonidine Hydrochloride Sustained Release Small Tablets
[0074] Component Dosage
[0075] Clonidine hydrochloride 0.1125
[0076] Hydroxypropyl methylcellulose K100 30
[0077] Kabob 9
[0078] Lactose 10.6375
[0079] Magnesium stearate 0.25
[0080] The preparation method is the same as in Example 1.
Embodiment 3
[0082] The consumption of present embodiment is the amount used by 1000
[0083] a. Hydrochlorothiazide immediate-release small tablets
[0084] Component Dosage
[0085] Hydrochlorothiazide 6
[0086] Microcrystalline Cellulose 10
[0087] Lactose 12.95
[0088] Crospovidone 0.9
[0089] Magnesium stearate 0.15
[0090] b. Hydrochlorothiazide extended-release small tablets
[0091] Component Dosage
[0092] Hydrochlorothiazide 31.5
[0093] Hydroxypropyl methylcellulose K15 7.8
[0094] Carbopol 1.2
[0095] Lactose 19.2
[0096] Magnesium stearate 0.3
[0097] c. Clonidine Hydrochloride Sustained Release Small Tablets
[0098] Component Dosage
[0099] Clonidine hydrochloride 0.1125
[0100] Hydroxypropyl Methyl Cellulose K15 36
[0101] Kabob 21
[0102] Lactose 2.5875
[0103] Magnesium stearate 0.3
[0104] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com