Method for preparing trinexapac-ethyl
A technology of trinexapac-ethyl and diethyl maleate, which is applied in the field of preparation of cyclohexanedicarboxylic acid high-efficiency plant growth regulator trinexapac-ethyl, can solve the problem of high toxicity of metal cyanide, increase of production cost, and unfavorable environmental protection and other problems, to achieve the effect of good product purity, reduced production cost, and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
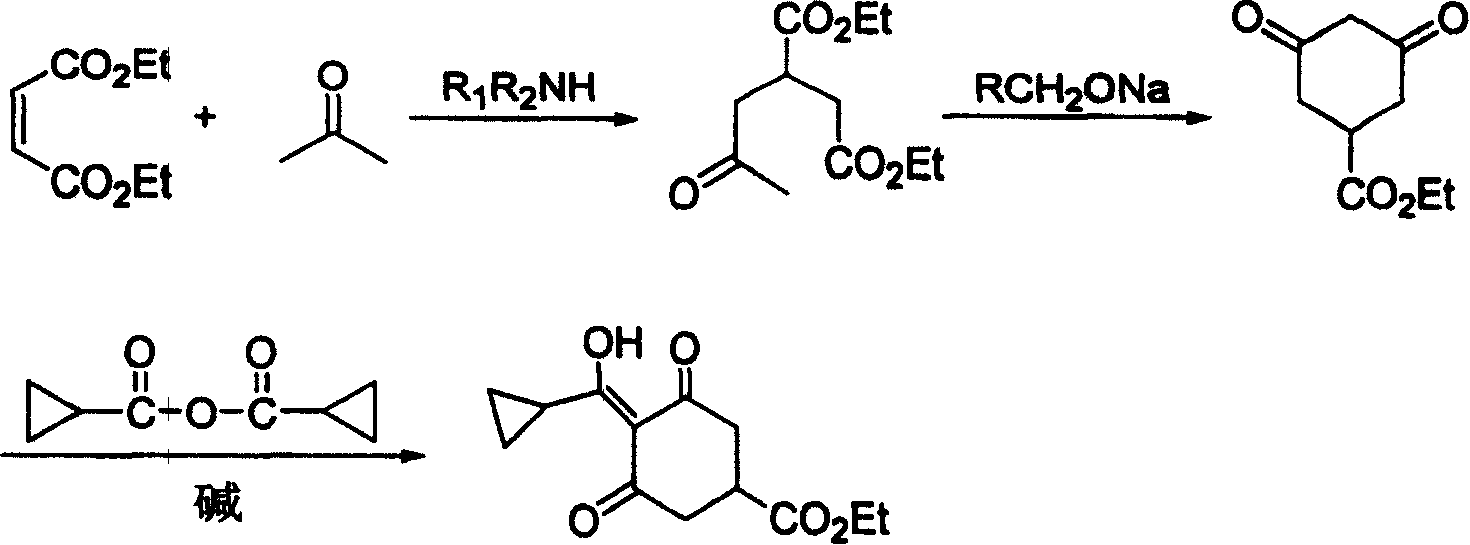
[0017] 1) Preparation of 2-acetonyl-1,4-diethyl succinate
[0018] Diethyl maleate (34.4g, 0.2mol), acetone (95.3g, 1.64mol) and dimethylamine (1.8g, 0.04mol) were dropped into a 500ml autoclave, heated to 150°C, and the pressure in the kettle was 10atm, and the temperature was kept React for 21 hours, cool to room temperature, remove low boilers at normal pressure, then distill under reduced pressure, collect fractions at 155-160°C / 5mmHg to obtain 39.8g of light yellow liquid with a yield of 86.5% and a content of more than 99%.
[0019] 2) Preparation of ethyl 3,5-cyclohexanedione-1-carboxylate
[0020] Drop into 40ml of 28% methanol solution of sodium methylate (10.8g, 0.056mol) in the reaction bottle, add dropwise 2-acetonyl-1,4-diethyl succinate (12g, 0.052mol), and heat to reflux after the dropwise addition After reacting for 5 hours, the reaction solution was cooled, concentrated to recover methanol, 80ml of water was added to the residue, extracted twice with 50ml of ...
Embodiment 2
[0024] 1) Preparation of 2-acetonyl-1,4-diethyl succinate
[0025] Diethyl maleate (34.4g, 0.2mol), acetone (95.3g, 1.64mol) and trimethylamine (2.4g, 0.041mol) were put into a 500ml autoclave, heated to 140°C, the pressure in the kettle was 15atm, and the reaction After 18 hours, cool to room temperature, remove low boilers at normal pressure, and then distill under reduced pressure to collect fractions at 155-160°C / 5mmHg to obtain 39.6g of light yellow liquid with a yield of 86.1% and a content of more than 99%.
[0026] 2) Preparation of ethyl 3,5-cyclohexanedione-1-carboxylate
[0027] Drop into 40ml of 15% sodium ethylate (25g, 0.055mol) ethanol solution in the reaction bottle, add dropwise 2-acetonyl-1,4-diethyl succinate (12g, 0.052mol), and heat to reflux after the dropwise addition After 5 hours, the reaction solution was cooled, concentrated to recover ethanol, 80ml of water was added to the residue, extracted twice with 50ml of dichloroethane to remove impurities, ...
Embodiment 3
[0031] 1) Preparation of 2-acetonyl-1,4-diethyl succinate
[0032] Diethyl maleate (34.4g, 0.2mol), acetone (95.3g, 1.64mol) and diethylamine (2.9g, 0.04mol) were put into a 500ml autoclave, heated to 160°C, the pressure in the kettle was 6atm, and the temperature was kept React for 24 hours, cool to room temperature, remove low boilers at normal pressure, then distill under reduced pressure, collect fractions at 155-160°C / 5mmHg to obtain 38.9g of light yellow liquid with a yield of 84.6% and a content of more than 99%.
[0033] Preparation of ethyl 3,5-cyclohexanedione-1-carboxylate
[0034] 40ml of 10% sodium tert-butoxide (51.8g, 0.054mol) in tert-butanol solution was dropped into the reaction flask, and 2-acetonyl-1,4-diethyl succinate (12g, 0.052mol) was added dropwise. After completion, the reaction was heated under reflux for 5 hours, the reaction solution was cooled, concentrated to recover tert-butanol, 80ml of water was added to the residue, and 50ml of dichloroetha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com