Method and devices for laser induced fluorescence attenuation spectroscopy
a laser and fluorescence attenuation technology, applied in the direction of luminescent dosimeters, optical radiation measurement, diagnostics using spectroscopy, etc., can solve the problems of difficult to perform attenuation measurements on certain types of samples, and limited material application of techniques, etc., to achieve the effect of maximum signal-to-noise ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
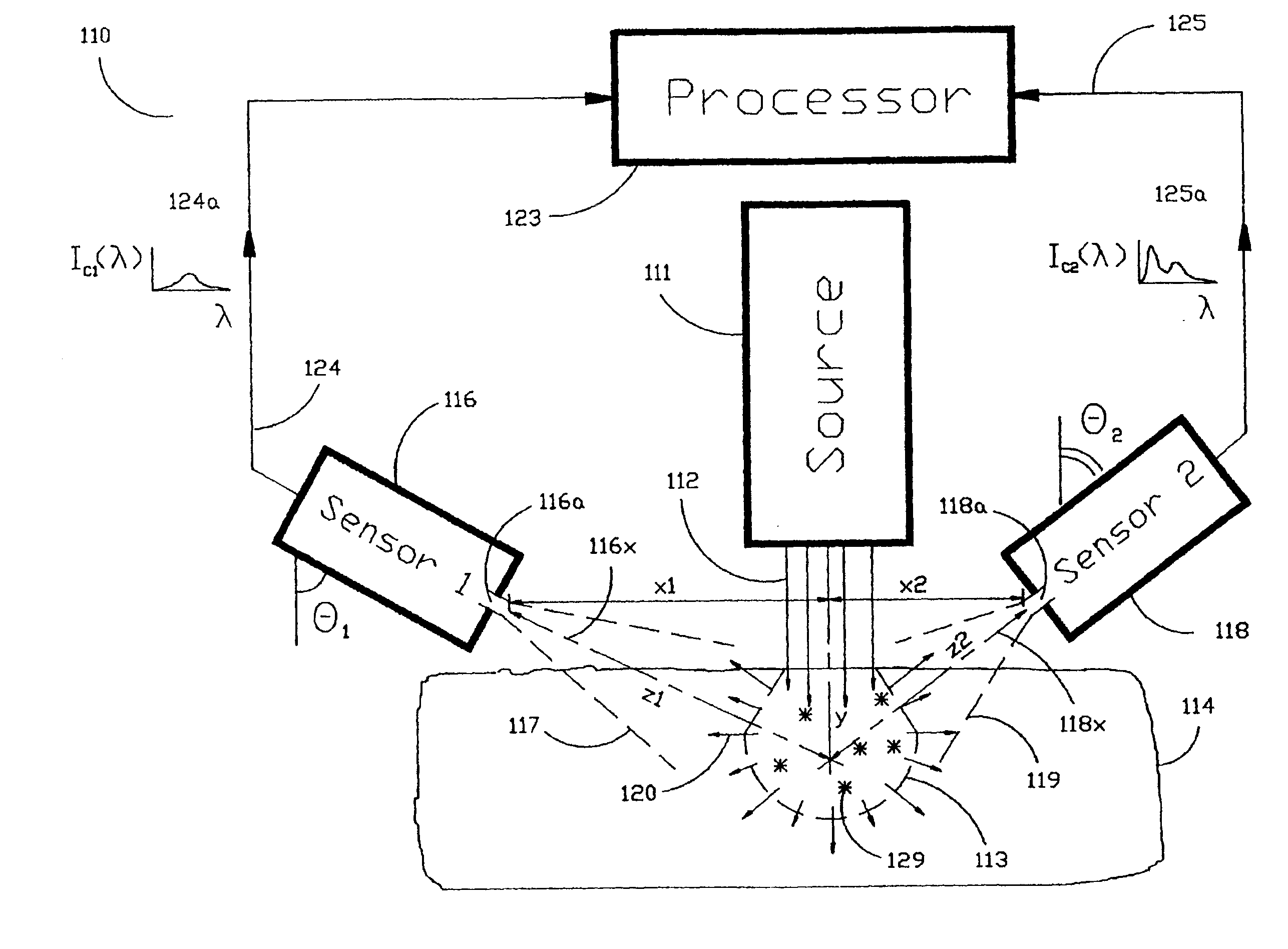
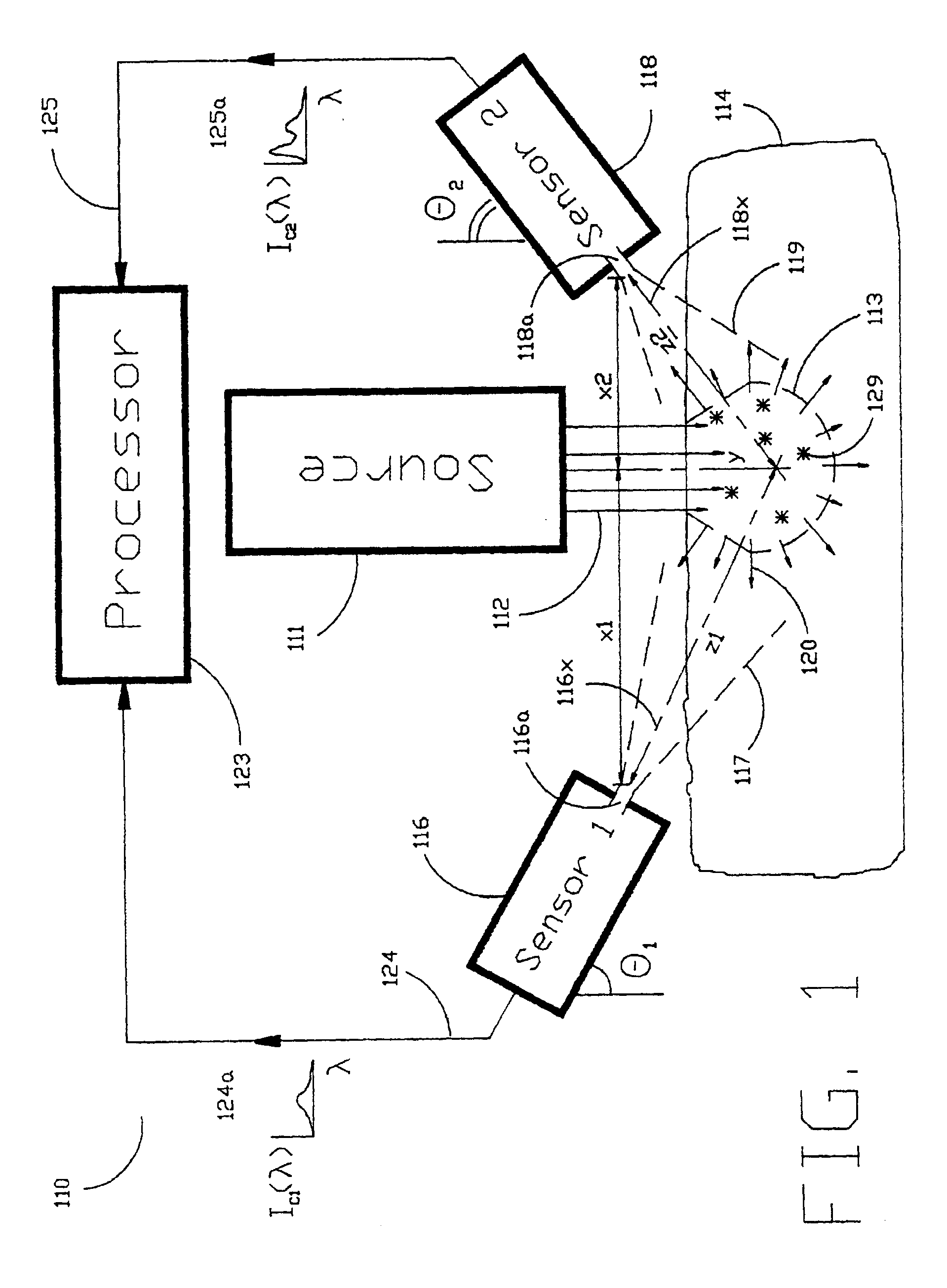
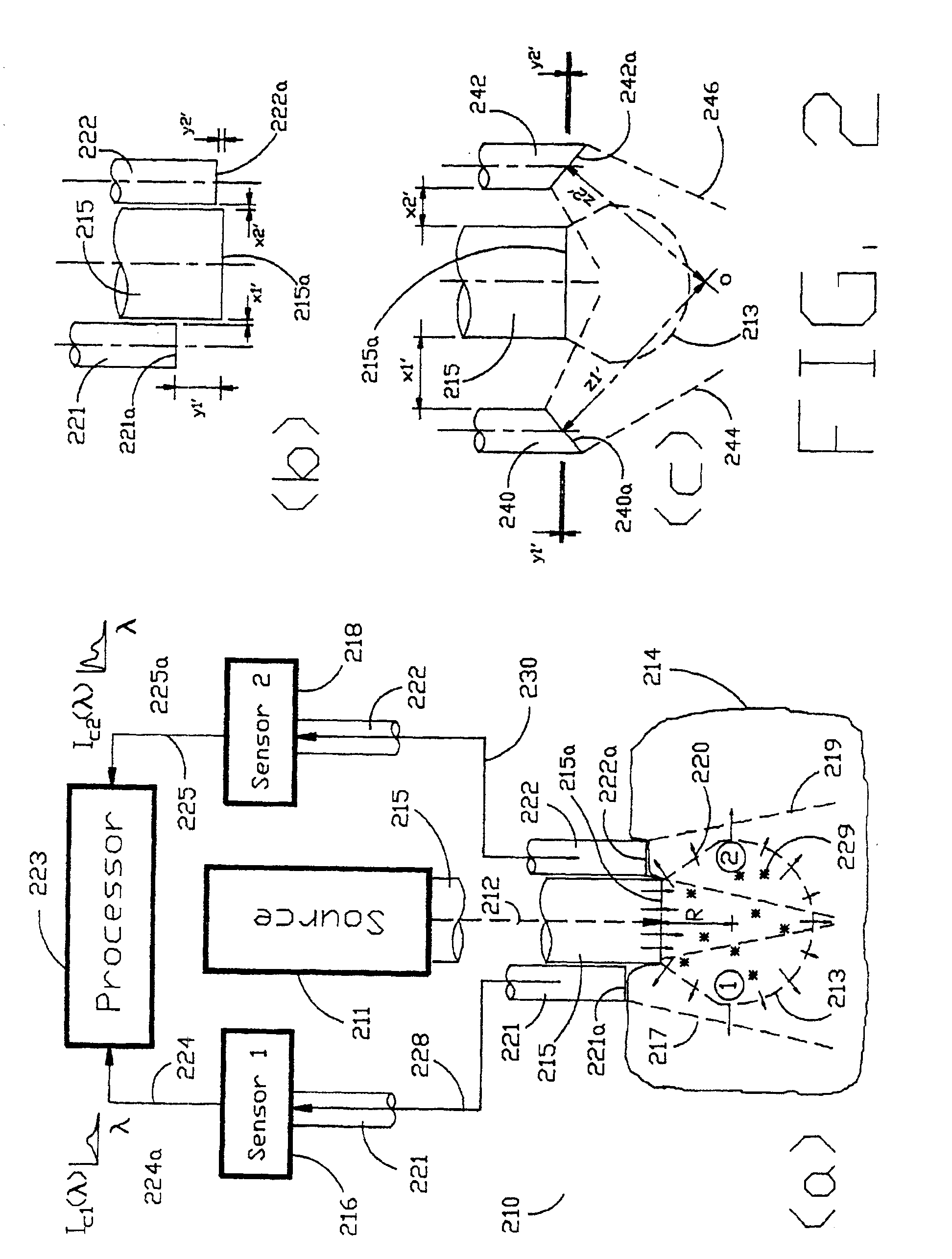
[0040]As shown in the exemplary drawings and, in particular, FIG. 1 thereof, the present invention is embodied in a spectroscopic system 110 and related method for measuring the attenuation and / or optical rotation caused by a sample 114. The spectroscopic system 110 shown in FIG. 1, includes a source 111 that produces radiation 112, which is directed at a sample volume 113 within the sample 114. The source 111 is preferably a laser and the radiation 112 is preferably monochromatic ultraviolet (UV), visible or infrared (IR) radiation 112. Nevertheless, the source 111 can be selected to produce other types of radiation, such as broadband or polarized radiation, useful for a particular application. The spectroscopic system 110 further includes a first sensor 116 displaced by a distance Z1 from the sample volume 113, and a second sensor 118 displaced by a distance Z2 from the sample volume 113, which are used to monitor return radiation 120 from the sample 114. In the preferred embodime...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| axial distance y2 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com