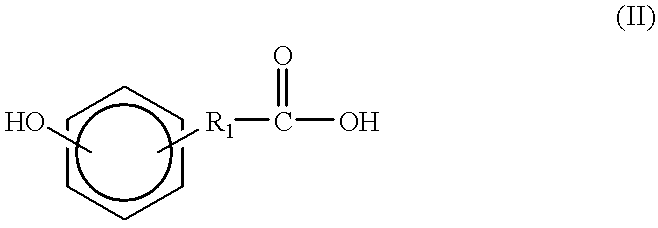
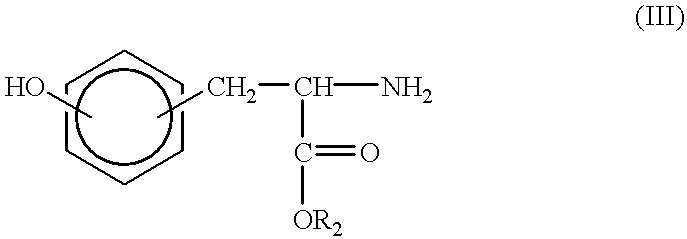Synthesis of tyrosine derived diphenol monomers
a technology of tyrosine derived diphenol and monomer, which is applied in the preparation of carboxylic acid amides, organic chemistry, chemistry apparatus and processes, etc., can solve the problems of incomplete difficult purification of l-tyrosine derived diphenols, and inability to achieve the complete removal of dcu by extraction and/or precipitation techniques, etc., to achieve the purity and yield of l-tyrosin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
PREPARATION OF DTH
Tyrosine hexyl ester (9.63 g, 36.3 mmol) and Dat (6.04 g, 36.3 mmol) were placed in a three-necked round bottom flask with a magnetic stir bar. The flask was sealed and flushed with nitrogen. 60 mL of freshly distilled THF was added by syringe. The reaction mixture was chilled in an ice bath for 10 minutes. Then EDCI.cndot.HCL (7.67 g, 40.0 mmol) was added while the nitrogen blanket was maintained. The reaction mixture stirred for one hour in an ice bath and for 19 hours at room temperature.
The reaction mixture was poured with stirring into 600 mL of water. An oil formed, which was removed by extraction into 120 mL of methylene chloride. The organic layer was washed with two portions of 200 mL of 0.1M Na.sub.2 CO.sub.3, two portions of 200 mL of saturated NaCl, two portions of 200 mL of 0.1M of citric acid, and two portions of 200 mL of saturated NaCl. All aqueous layers were backwashed with 10 mL of methylene chloride. The organic phases were combined, dried over ...
example 2
PREPARATION OF DTE
The procedure of Example 1 was repeated, substituting tyrosine ethyl ester (4.00 g, 19.0 mmol) for the tyrosine hexyl ester. Ethyl acetate was substituted for methylene chloride as the extraction solvent. Product yield and purity are listed in Table II. Elemental analysis: calculated: 67.0% C, 6.8% H, 3.9% N; experimental: 66.9%, C, 6.6% H, 3.7% N. .sup.1 H-NMR (CDCl.sub.3): 1.24 (t, 3H), 2.45 (m, 2H), 2.95 (m, 4H), 4.20 (q, 2H), 4.83 (q, 1H), 5.90 (d, 1H), 6.70 (m, 6H), 7.00 (d, 2H).
example 3
PREPARATION OF DTB
The procedure of Example 1 was repeated, substituting tyrosine butyl ester (7.50 g, 31.6 mmol) for the tyrosine hexyl ester. Product yield and purity are listed in Table II. Elemental analysis: calculated: 68.4% C, 7.3% H, 3.6% N; experimental: 68.5% C, 7.2% H, 3.4% N. .sup.1 H-NMR (CDCl.sub.3): 0.930 (t, 3H), 1.30 (m, 2H), 1.64 (m, 2H), 2.46 (m, 2H), 2.90 (m, 4H), 4.10 (t, 2H), 4.80 (q, 1H), 5.90 (d, 1H), 6.70 (m, 6H), 6.95 (d, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| weight-average molecular weights | aaaaa | aaaaa |
| weight-average molecular weights | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com