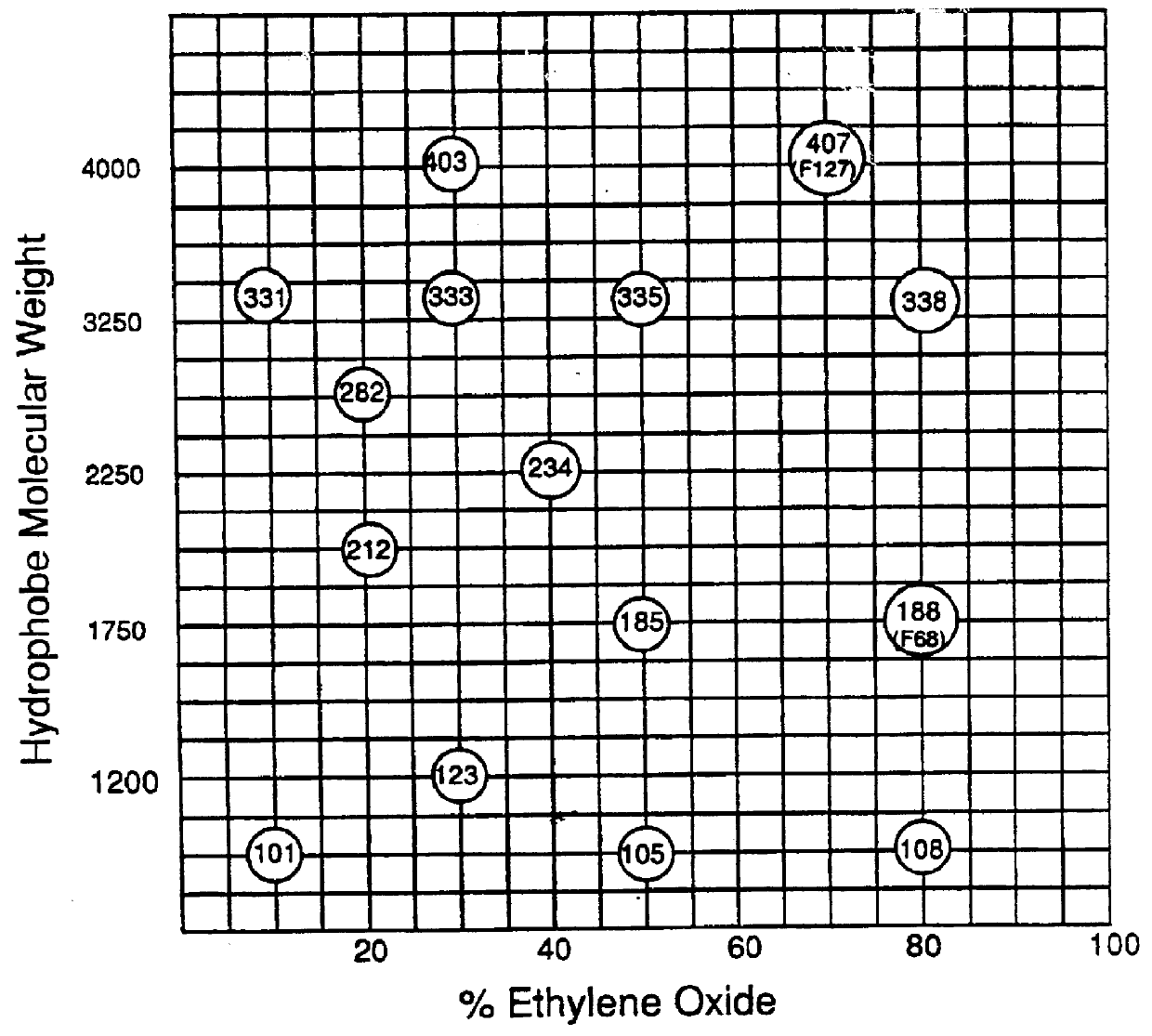
Polyoxypropylene/polyoxyethylene copolymers with improved biological activity
a polyoxyethylene and polyethylene technology, applied in the field of polyoxypropylene/polyoxyethylene copolymer preparation, can solve the problems of toxic to cells, unsatisfactory biological activity of the surface-active copolymer, and low molecular weight molecules with unsaturated polymers in the population, so as to achieve better biological activity and more predictable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
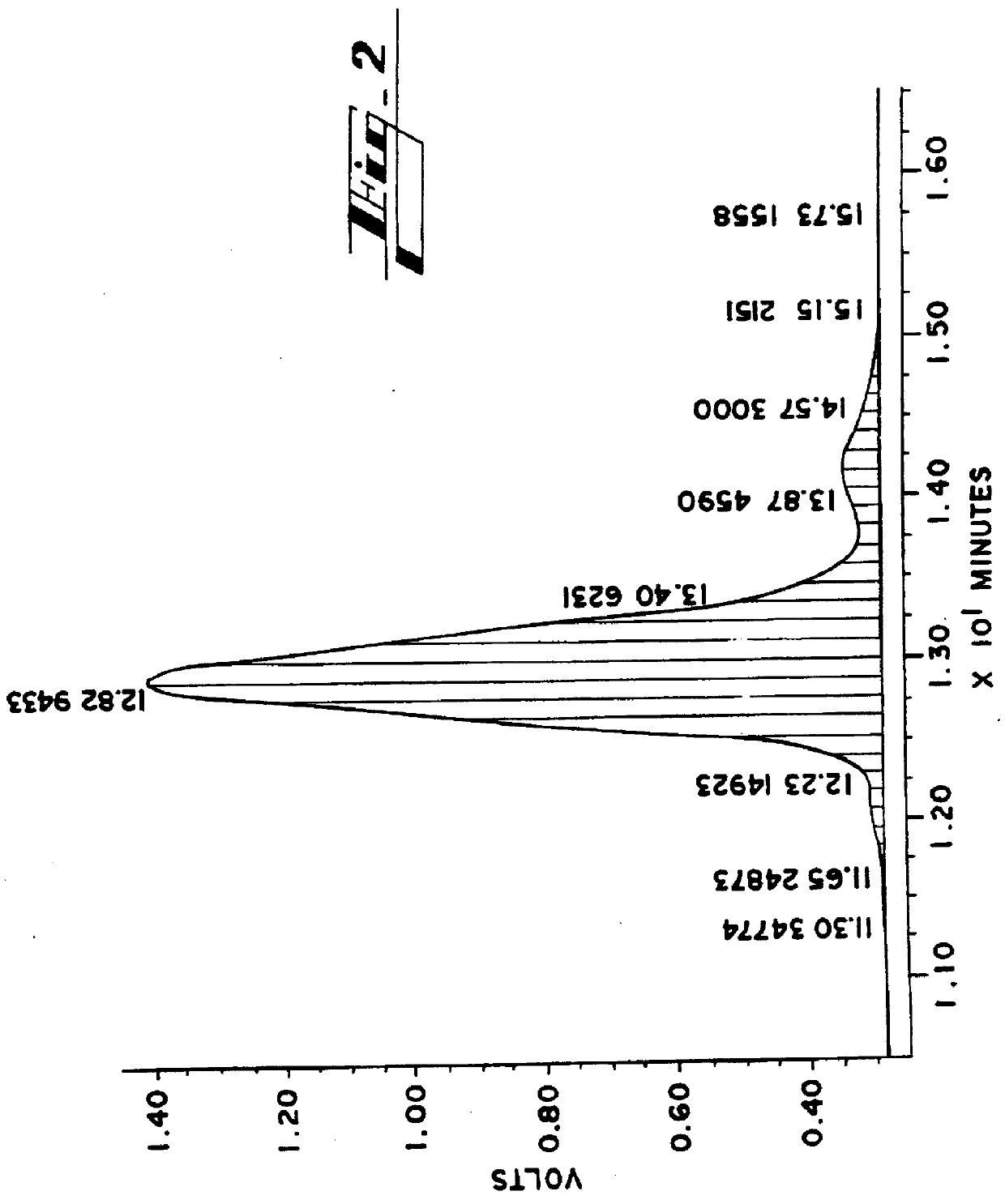
Poloxamer 188 (BASF Corporation, Parsippany N.J.) is dissolved in tetrahydrofuran at a concentration of 20 mg / mL. A Model 600E Powerline chromatographic system equipped with a column heater module, a Model 410 refractive index detector and Maxima 820 software package (all from Waters, Div. of Millipore, Milford, Mass.) is used to fractionate the commercially prepared poloxamer 188 copolymer. The chromatographic system is equipped with two LiChrogel PS-40 columns and a LiChrogel PS-20 column in series (EM Science, Gibbstown, N.J.). The LiChrogel PS-40 columns are 10 .mu.m particle size and the LiChrogel PS-20 column is 5 .mu.m particle size. All columns are 7 mm by 25 cm in size.
200 .mu.L (4 mg) of the poloxamer 188 in tetrahydrofuran is added to the column and the sample is run with the columns and the detector at 40.degree. C. The resulting chromatogram is shown in FIG. 2.
example ii
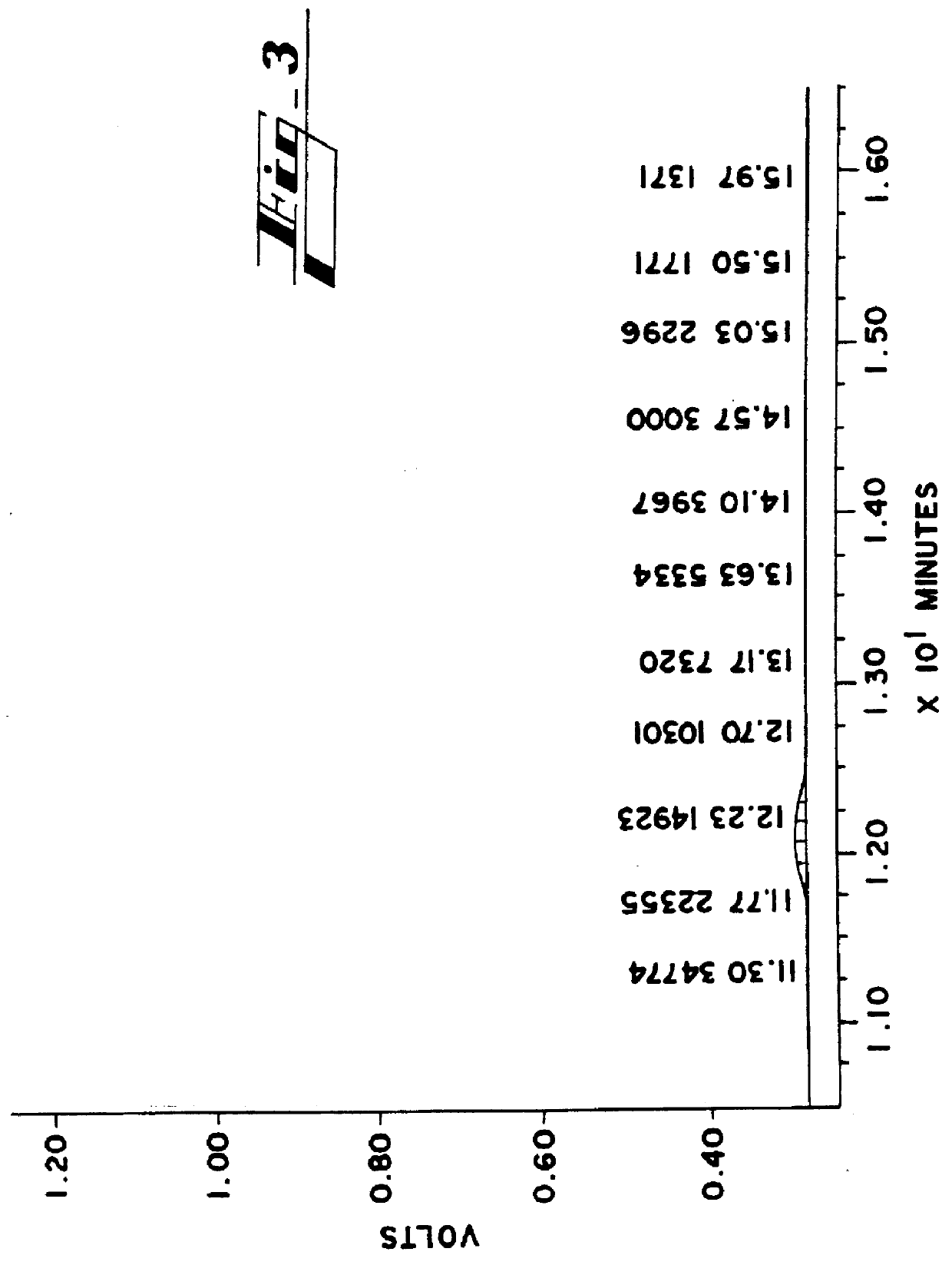
The sample that was collected in Example I was fractionated into six fractions and each fraction was run on the column as described in Example I. The chromatograms from the various chromatographic runs are shown in FIGS. 3 through 8. The fraction that demonstrates the least toxicity while retaining the therapeutic activity of the poloxamer 188 is shown in FIG. 5. As can be clearly seen, the shoulders on either side of the peak in FIG. 5 are absent.
The average molecular weight for each fraction is shown in Table II. The chromatogram for each fraction is indicated in FIGS. 3 through 8.
TABLE II ______________________________________ Time off Molecular Polydispersity Fraction FIG. Column (Min) Wt. Value ______________________________________ 1 3 11.5-12.0 17000 1.0400 2 4 12.0-12.5 10270 1.0474 3 5 12.5-13.0 8964 1.0280 4 6 13.0-13.5 8188 1.0332 5 7 13.5-14.0 5418 1.1103 6 8 14.0-14.5 3589 1.0459 ______________________________________
The polydispersity value for the unfractionated polox...
example iii
In a one-liter 3 neck round bottom flask equipped with a mechanical stirrer, reflux condenser, thermometer and propylene oxide feed inlet, there is placed 57 grams (0.75 mol) of propylene glycol and 7.5 grams of anhydrous sodium hydroxide. The flask is purged with nitrogen to remove air and heated to 120.degree. C. with stirring until the sodium hydroxide is dissolved. Sufficient propylene oxide is introduced into the mixture as fast as it reacts until the product possesses a calculated molecular weight of approximately 1750 daltons. The product is cooled under nitrogen and the NaOH catalyst is neutralized with sulfuric acid and the product is then filtered. The final product is a water-insoluble polyoxypropylene glycol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com