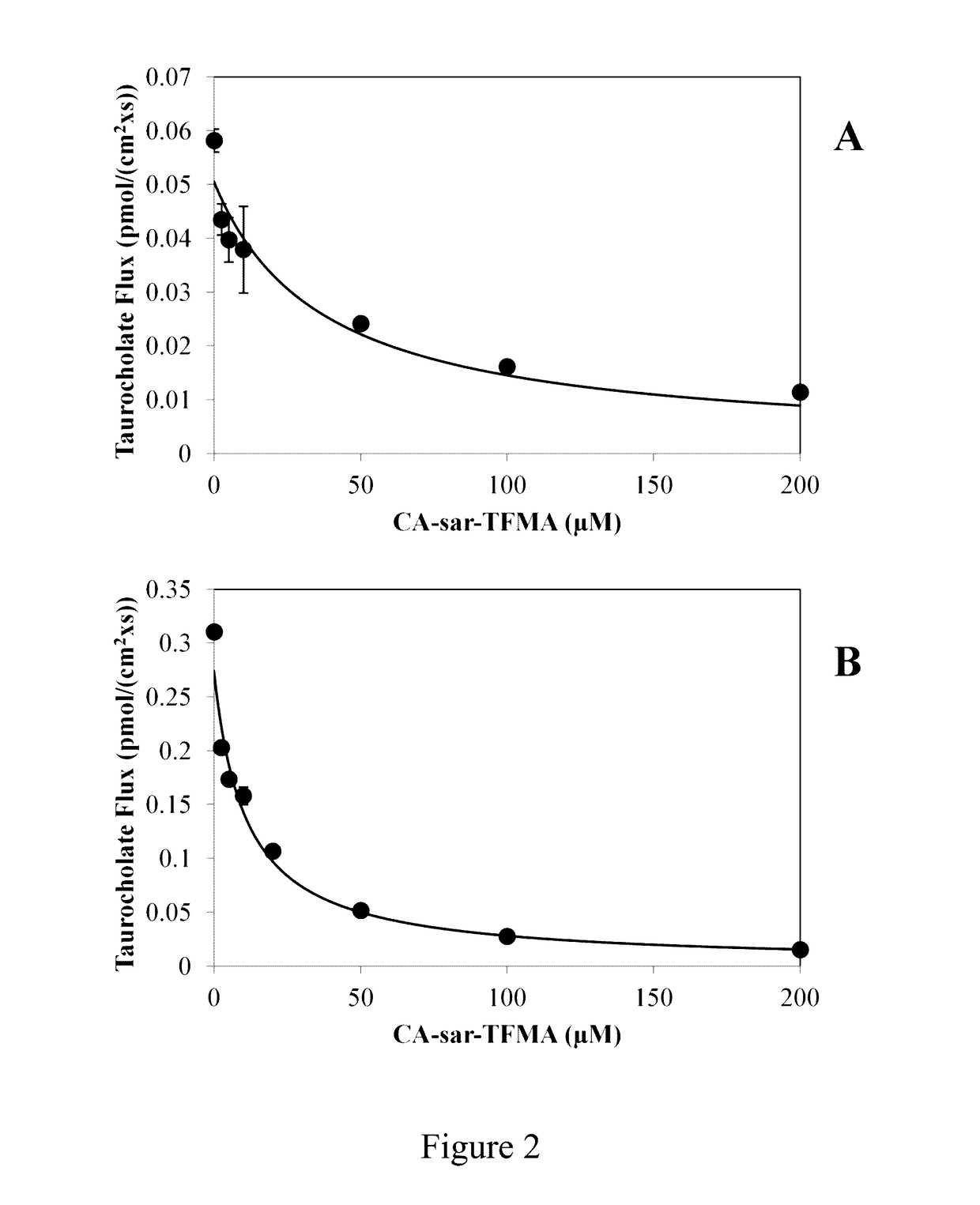
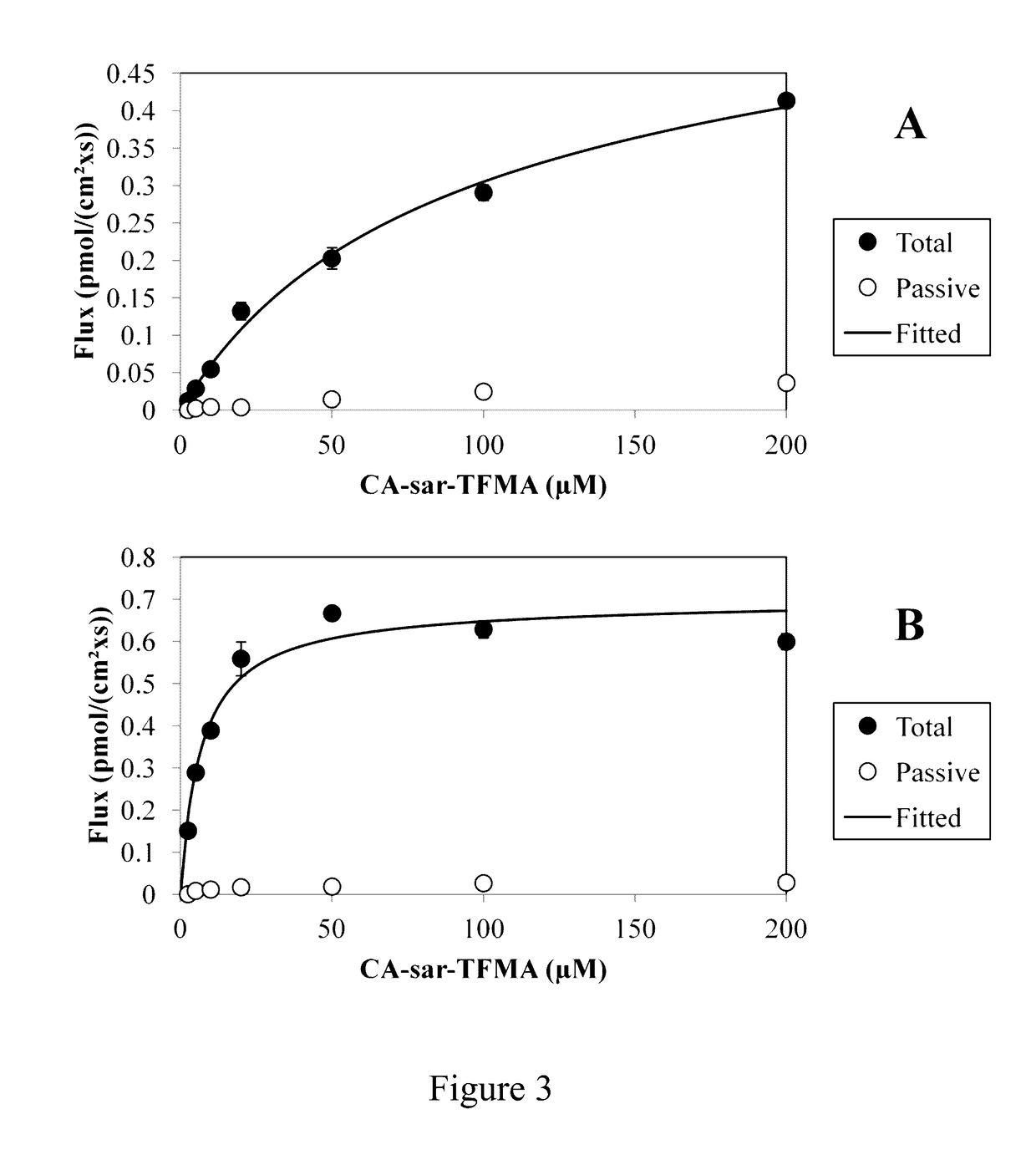
Compositions and methods to evaluate hepatobiliary/gastrointestinal health, enterohepatic circulation, and drug interactions
a technology of hepatobiliary/gastrointestinal health and composition, applied in the field of detection probes, can solve the problems of inability to detect bam, limited diagnosis, sensitive, specific and cost-effective tests, etc., and achieve the effect of improving the effectiveness of magnetic resonance imaging (mri) of a patient's gallbladder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials
[0059]Taurocholate, cholic acid, trifluoroacetic anhydride, rat liver S9 fraction, trifluoroacetic acid, rat plasma, and choloylglycine hydrolase from Clostridium perfringens were obtained from Sigma Aldrich (St. Louis, Mo.). N-boc-ethylene diamine was purchased from Oakwood Chemical (West Columbia, S.C.). [3H]-taurocholate (10 μCi / mM) was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, Mo.). Trypsin, geneticin, fetal bovine serum (FBS) and Dulbecco's modified Eagle medium (DMEM) were purchased from Invitrogen (Rockville, Md.). All other reagents and chemicals were of the highest purity available commercially.
[0060]Methods
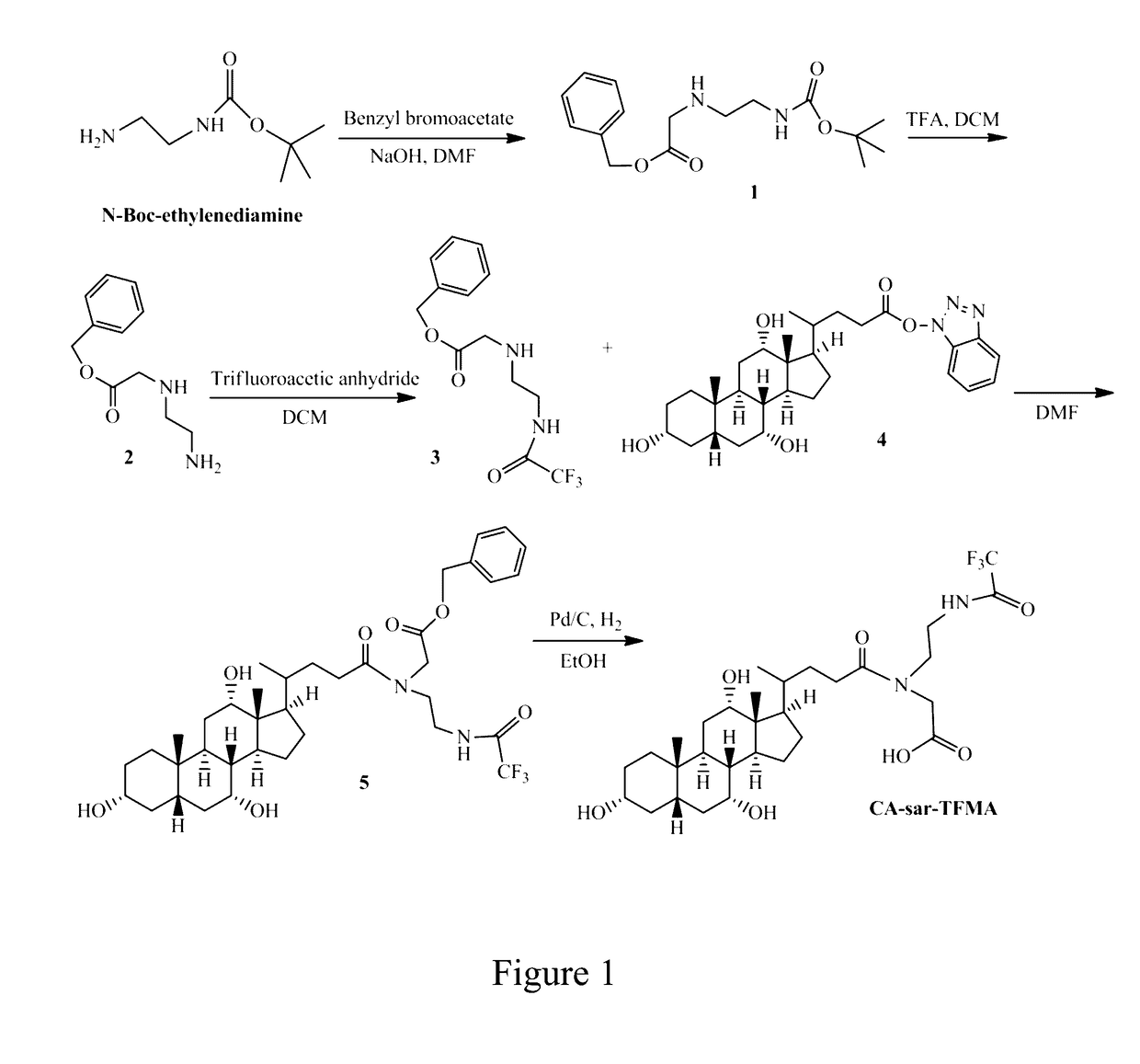
[0061]Synthesis of CA-sar-TFMA
[0062]CA-sar-TFMA was synthesized as in FIG. 1. 2 mL (12.6 mmol) N-boc-ethylene diamine was stirred for 15 min with 2 eq. (25.2 mmol) sodium hydroxide (NaOH) in dimethyl formamide (DMF). To this mixture, 0.6 eq. (7.6 mmol) benzyl bromoacetate was added and stirred overnight at room temperature. DMF was diluted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com