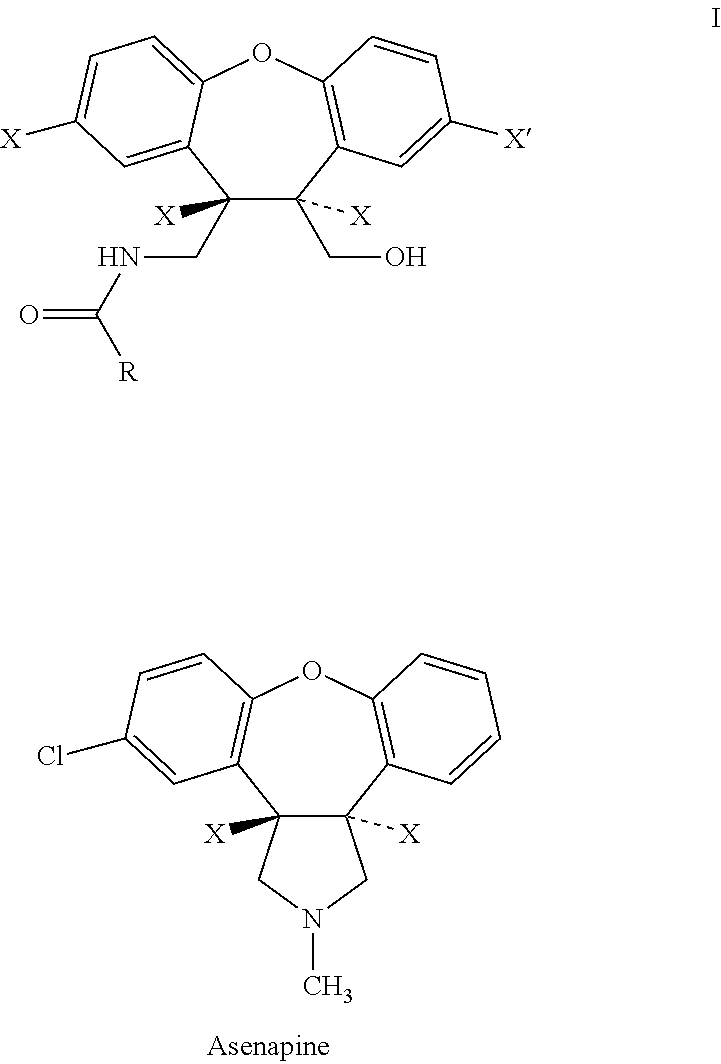
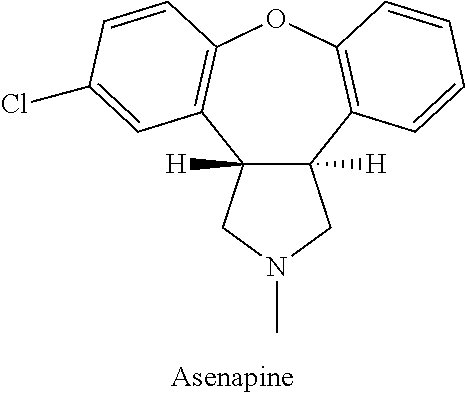
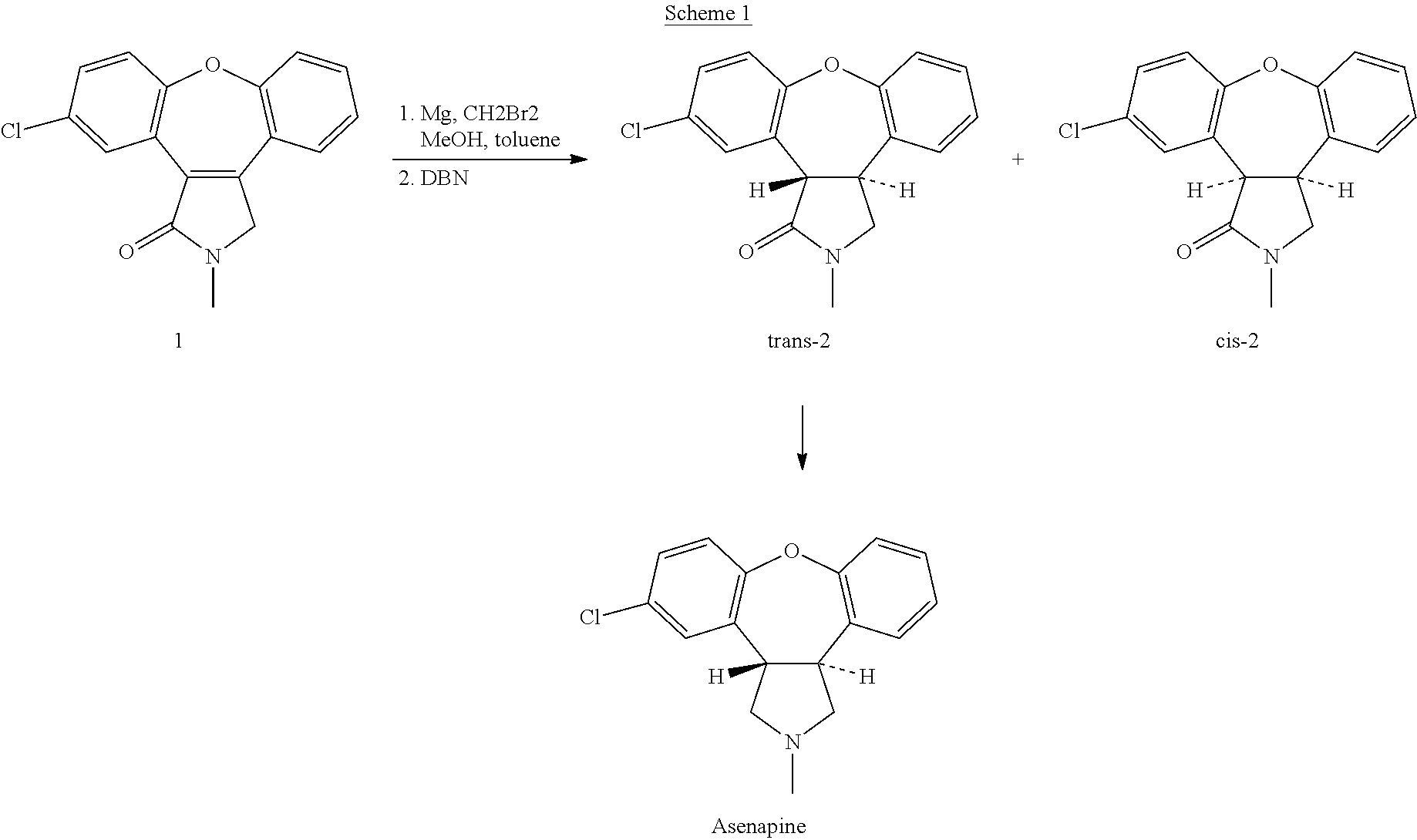
Process for the preparation of asenapine
a technology of asenapine and process, which is applied in the field of new compounds of formula i, can solve the problems of only moderate final yield of trans-isomer, complex and time-consuming, etc., and achieve good esteroselectivity and yield. good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of trans-(11-Aminomethyl-2-chloro-10,11-dihydro-dibenzo[b,f]oxepin-10-yl)-methanol (5)
[0132]
[0133]A solution of trans-2-Chloro-11-nitromethyl-10,11-dihydro-dibenzo[b,f]oxepine-10-carboxylic acid methyl ester (4) (4.6 g, 13.23 mmol) in dry THF (23 ml) is added at—15° C. to a mixture of THF (23 ml) and 3.5 M Lithium aluminum hydride (LAH) suspension in THF / Toluene (15.1 ml, 52.9 mmol).
[0134]The mixture is stirred at 30° C. for 30 minutes, cooled to −15° C. and sequentially quenched with H2O (2 ml), 15% NaOH (2 ml) and H2O (6 ml).
[0135]The solid is filtered, washed with THF (2×23 ml) and the filtrate evaporated to dryness to give 3.60 g (95%) of trans-(11-Aminomethyl-2-chloro-10,11-dihydro-dibenzo[b,f]oxepin-10-yl)-methanol (5) as a pale yellow solid.
[0136]1-H-RMN (CDCl3, 200 MHz): 1.64 (br s, 3H, exchg. D2O), 2.70-2.80 (m, 1H) 2.87-2.97 (m, 1H), 3.12-3.18 (m, 1H) 3.19-3.36 (m, 1H), 3.44-3.54 (m, 1H), 3.63-3.72 (m, 1H), 7.03-7.26 (m, 7H).
example 2
Preparation of trans-N-(8-Chloro-11-hydroxymethyl-10,11-dihydro-dibenzo[b,f]oxepin-10-ylmethyl)-formamide (6)
[0137]
[0138]A mixture of Acetic Anhydride (2 ml, 20.7 mmol) and Formic Acid (1.6 ml, 41.4 mmol) is heated to 50° C. for 2 hours. After cooling to 25° C. the mixture is diluted with dichloromethane (15 ml). Next, reaction is cooled to 0° C. and trans-(11-Aminomethyl-2-chloro-10,11-dihydro-dibenzo[b,f]oxepin-10-yl)-methanol (5) (3.00 g, 10.4 mmol) is added and is stirred at 25° C. for one hour.
[0139]Reaction is quenched with 10% K2CO3 (20 ml) and organic layer is washed with 10% K2CO3 until pH 9.
[0140]Methanol (3 ml) and solid K2CO3 (0.72 g, 5.21 mmol) are added to the organic layer and stirred for 2 hours at room temperature (r.t.). Water (30 ml) is then added and stirred for additional 15 min. Organic layer is then separated, washed with water (2×20 ml) and brine (20 ml) and evaporated to dryness to give 2.45 g (76%) of trans-N-(8-Chloro-11-hydroxymethyl-10,11-dihydro-dibenzo...
example 3
Preparation of trans-(2-Chloro-11-methylaminomethyl-10,11-dihydro-dibenzo[b,f]oxepin-10-yl)-methanol (7)
[0142]
[0143]Sodium Borohydride (0.80 g, 21.2 mmol) is added at 0° C. to a solution of trans-N-(8-Chloro-11-hydroxymethyl-10,11-dihydro-dibenzo[b,f]oxepin-10-ylmethyl)-formamide (6) (2.25 g, 7.1 mmol) in dry THF (15 ml). The mixture is stirred for 10′. Next Boron trifluoride tetrahydrofuran complex (4 ml, 34.6 mmol) is added dropwise maintaining temperature below 5° C. Reaction is then stirred at 35° C. for 15 h.
[0144]Reaction is then cooled to 0° C. and 3N HCl (15 ml) is added, then is heated to 100° C. and stirred for 30 minutes, during heating about 15 ml of tetrahydrofuran are distilled.
[0145]Next is cooled to room temperature and 10% K2CO3 is added until pH 9, followed by ethyl acetate (30 ml). Organic layer is separated and washed with water, 1M NaOH and brine and evaporated to dryness to obtain 1.85 g (87%) of trans-(2-Chloro-11-methylaminomethyl-10,11-dihydro-dibenzo[b,f]ox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com