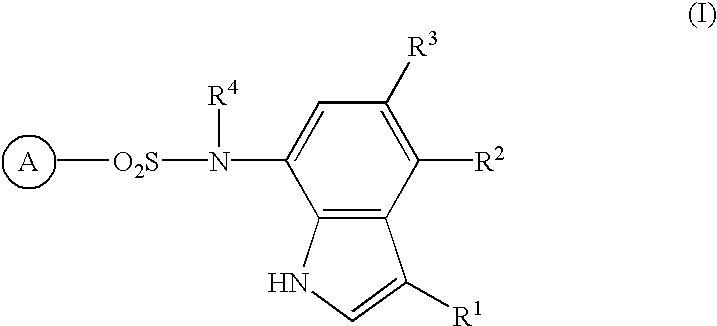
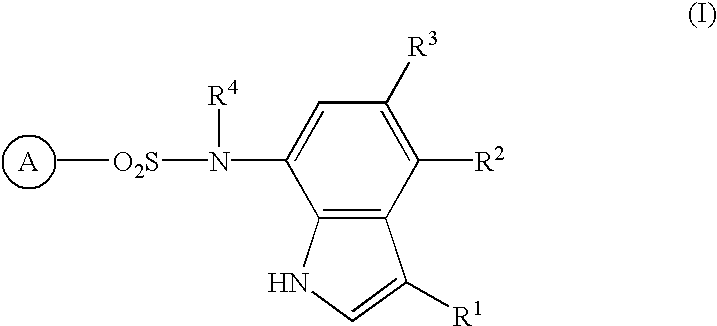
Sulfonamide-containing indole compounds
a technology of indole and sulfonamide, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, biocides, etc., can solve the problems of blindness, poor eye sight, and doubtful clinical usefulness of indole compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
production example 1
Ethyl pyruvate N-(5-methyl-2-nitrophpnyl)hydrazone
To a mixed solution of 160 ml of water and 170 ml of concentrated hydrochloric acid was added 75.0 g (493 mmol) of 5-methyl-2-nitroaniline followed by stirring. An aqueous solution (80 ml) of 36.0 g (517 mmol) of sodium nitrite was added dropwise thereinto at -20.degree. C. The reaction solution was added to a solution which was prepared by dissolving ethyl 2-methylacetacetate in 100 ml of ethanol followed by adding 200 ml of a 12N aqueous solution of potassium hydroxide, at -20.degree. C. with stirring during 30 minutes. After the mixture was stirred at the same temperature for 30 minutes, 100 ml of concentrated hydrochloric acid were added and the resulting precipitates were collected by filtration, washed with water and dried in vacuo for overnight. A mixed solution of diethyl ether and hexane was added thereto, and the resulting crystals were collected by filtration to give 130 g of the title compound.
.sup.1 H-NMR (DMSO-d.sub.6) ...
production example 2
Ethyl 4-methyl-7-nitro-1H-indole-2-carboxylate
To 250 ml of a suspension of 25.0 g (94.2 mmol) of the compound of Production Example 1 in xylene was added 100 g of polyphosphoric acid followed by heating under reflux for 3 hours. To the reaction solution were added 80 ml of water and 300 ml of ethyl acetate under ice-cooling. The resulting insoluble matters were filtered off followed by washing with 1.5 liters of ethyl acetate, and the resulting filtrate was extracted with ethyl acetate. The organic layer was successively washed with a saturated sodium bicarbonate solution, water and brine, dried over magnesium sulfate and concentrated to dryness. To the resulting residue was added a mixed solution of tert-butyl methyl ether and hexane, and the resulting crystals were collected by filtration to give 11.1 g of the title compound.
.sup.1 H-NMR (DMSO-d.sub.6) .delta. (ppm); 1.35(3H, t, J=7.2 Hz), 2.65(3H, s), 4.38(2H, q, J=7.2 Hz), 7.16(1H, d, J=8.4 Hz), 7.51(1H, s), 8.19(1H, d, J=8.4 Hz...
production example 3
4-Methyl-7-nitron-1H-indole-2-carboxylic Acid
To 150 ml of a solution of 11.0 g (44.3 mmol) of the compound of Production Example 2 in tetrahydrofuran was added 150 ml of a 1N aqueous solution of sodium hydroxide followed by heating under stirring at 80.degree. C. for 30 minutes. The reaction solution was concentrated, 40 ml of 5N hydrochloric acid was added to the resulting residue under ice-cooling to adjust to pH 1, and the resulting precipitates were filtered and washed with water. The precipitates were dissolved in 300 ml of tetrahydrofuran and extracted with ethyl acetate. The organic layer was washed with brine, dried over magnesium sulfate and concentrated to dryness to give 9.60 g of the title compound.
.sup.1 H-NMR (DMSO-d.sub.6) .delta. (ppm); 2.62(3H, s), 7.13(1H, d, J=8.0 Hz), 7.42(1H, S), 8.15(1H, d, J=8.0 Hz), 11.00(1H, brs).
Prduction Example 4
4-Methyl-7-nitro-1H-indole
Into 60 ml of 1,3-dimethyl-2-imidazolidinone was dissolved 9.58 g (43.5 mmol) of the compound of Produ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wave length | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com