Anti-protozoal compounds and uses thereof
a technology of protozoal compounds and compounds, applied in the field of veterinary formulations, can solve the problems of significant impact on the breeding herd, endometritis, cervicitis, vaginitis, etc., and achieve the effect of improving administration and treatment, and reducing the risk of infection in the breeding herd
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
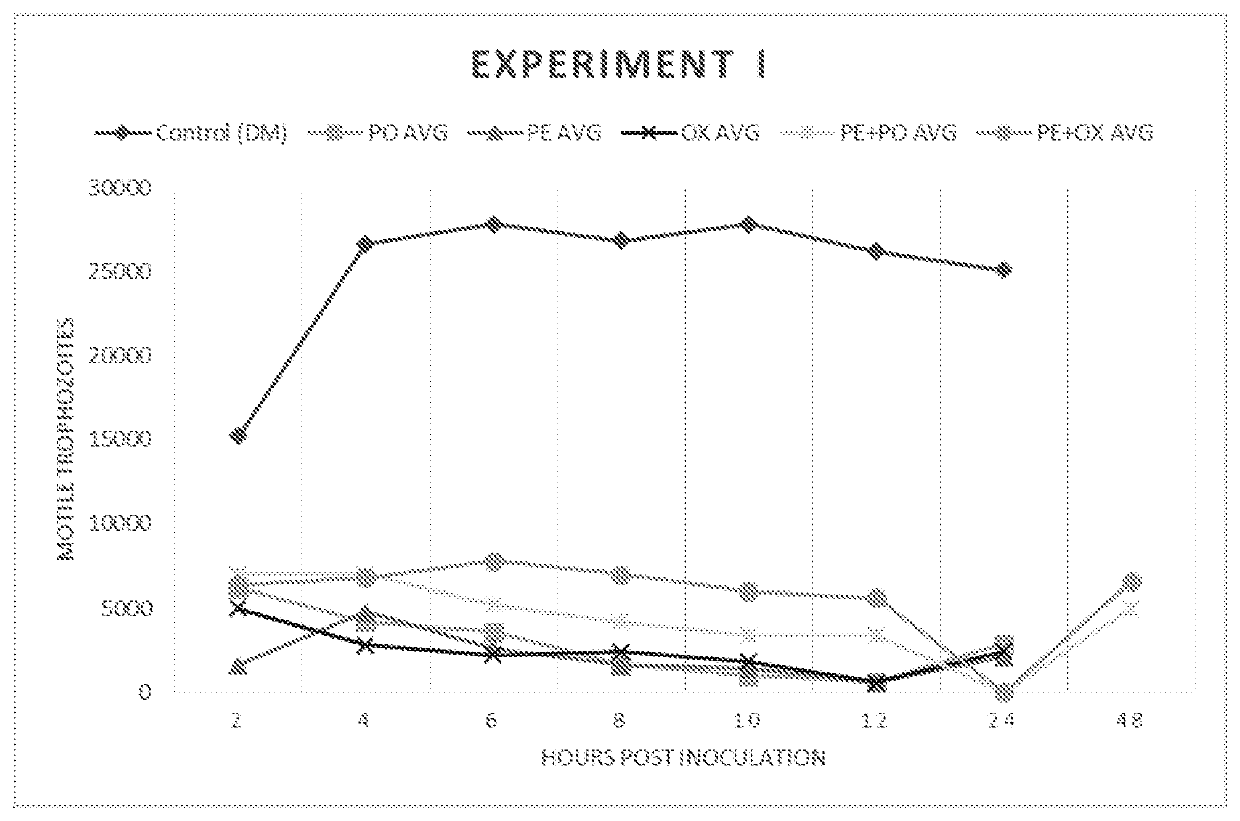
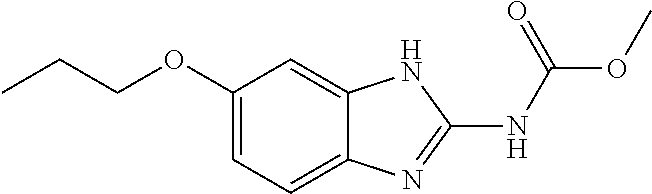
[0163]Trophozoites (3.75×105 mL−1) were washed in PBS (VWR, Radnor, Pa.) and inoculated into 10 mL of fresh DM. At time 0, each of the following treatment groups was added to culture tubes: Group 1) control: 0.5 mL DM; Group 2) 0.5 mL pluronic lecithin organogel (PLO; Pluronic F127); Group 3) 75 mg ponazuril (PO) (0.5 mL); Group 4) 50 mg oxibendazole (OX) (0.5 mL); Group 5) 37.5 mg PO (0.25 mL)+0.25 mL PLO; and Group 6) 25 mg OX (0.25 mL)+0.25 mL PLO.
[0164]PLO (Pluronic F127 Letco Medical, Decatur, Ala.) was combined with OX (Anthelcide EQ® Zoetis, Florham Park, N.J.) or PO (Marquis® Merial, Duluth, Ga.) paste formulations by shear force, syringe-to-syringe-blending using two luer-lock syringes and a rapid fill connector (Baxter, Inglewood, Calif.). Each treatment was applied to conical culture tubes in 5 replicates. Following growth at 37° C., samples were taken every 2 hours for a total of 12 hours, and the tubes were vortexed prior to removal of a 20 μL sample. From these samples...
example 2
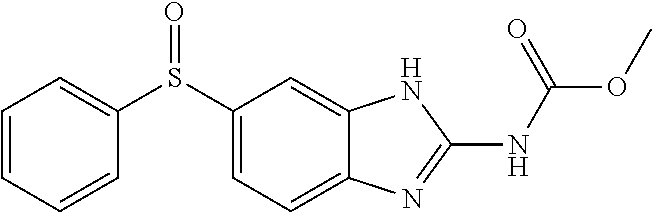
[0167]Trophozoites (9.5×105 mL−1) were cultured as described above and added to tissue culture wells with 3 mL of DM. At time 0, each of the following treatment groups were added to tissue culture wells: Group 1) control 4 mL DM; Group 2) 4 mL PLO; Group 3) 150 mg oxfendazole (OXF)+1 mL PLO; and Group 4) 450 mg PO (3 mL)+1 mL PLO.
[0168]The OXF (U.S. Pharmacopeial Convention Rockville, Md.) stock solution was made in EtOH (Humco Texarkana, TX) and mixed with Velvachol (“VC”; Valeant North America LLC, Bridgewater, N.J.) and thereafter was stored at 150 mg mL−1. PLO was combined with OXF stock solution or PO by shear force by syringe-to-syringe mixing. Each treatment was applied to tissue culture wells in duplicate. Following growth at 37° C. on a rocker plate (Hoefer Inc., Holliston, Mass.), samples were collected every 4 hours for a total of 24 hours. A 20 μL sample was removed from each well, and the surviving organisms were counted utilizing disposable Neubauer hemocytometers.
[016...
example 3
[0171]Trophozoites (5×104 mL−1) were cultured as described above and added to all tissue culture wells along with 3 mL of DM for a total volume of 4 mL of DM with 50,000 organisms per well. At time 0, each of the following treatment groups were added to tissue culture wells as follows: Group 1) positive control 4 mL DM; Group 2) negative control 4 mL 70% ethanol (EtOH); Group 3) 150 mg oxfendazole solution (OXF) (1 mL)+2 mL Velvachol (VC)+1 mL PLO; Group 4) 150 mg OXF (1 mL)+3 mL VC; Group 5) 150 mg OXF (1 mL)+3 mL EtOH; Group 6) 150 mg OXF (1 mL)+2 mL EtOH+1 mL PLO; Group 7) 1 mL EtOH+2 mL VC+1 mL PLO; Group 8) 1 mL EtOH+3 mL VC; Group 9) 3 mL EtOH+1 mL PLO; Group 10) 4 mL EtOH; Group 11) 4 mL VC; Group 12) 150 mg oxfendazole dissolved in 4 mL 99% DMSO; and Group 13) 4 mL 99% DMSO.
[0172]The OXF (U.S. Pharmacopeial Convention Rockville, Md.) stock solution was made in EtOH (Humco Texarkana, TX) and stored at 150 mg mL−1, with the exception of Formulation 13 which included OXF dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com