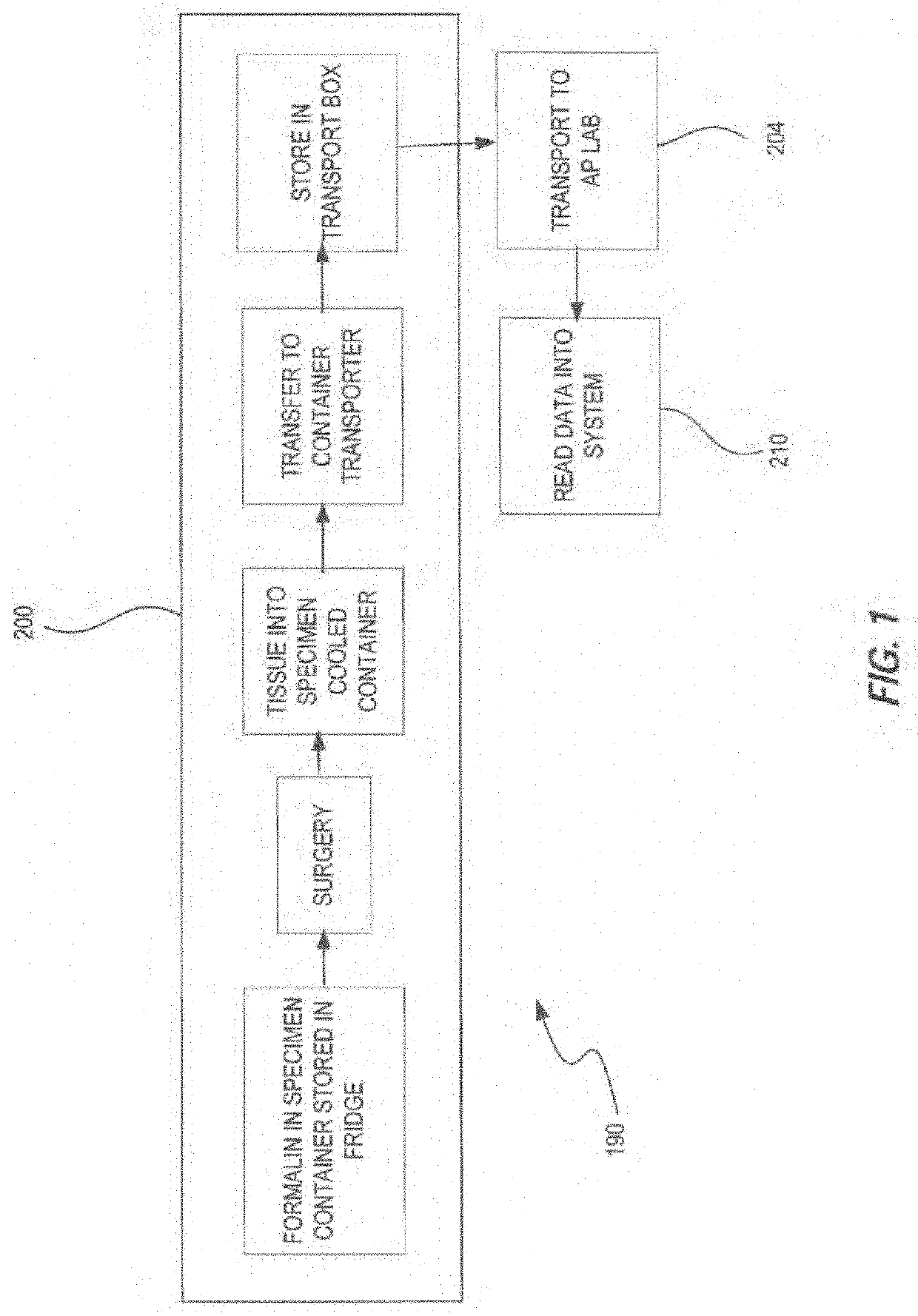
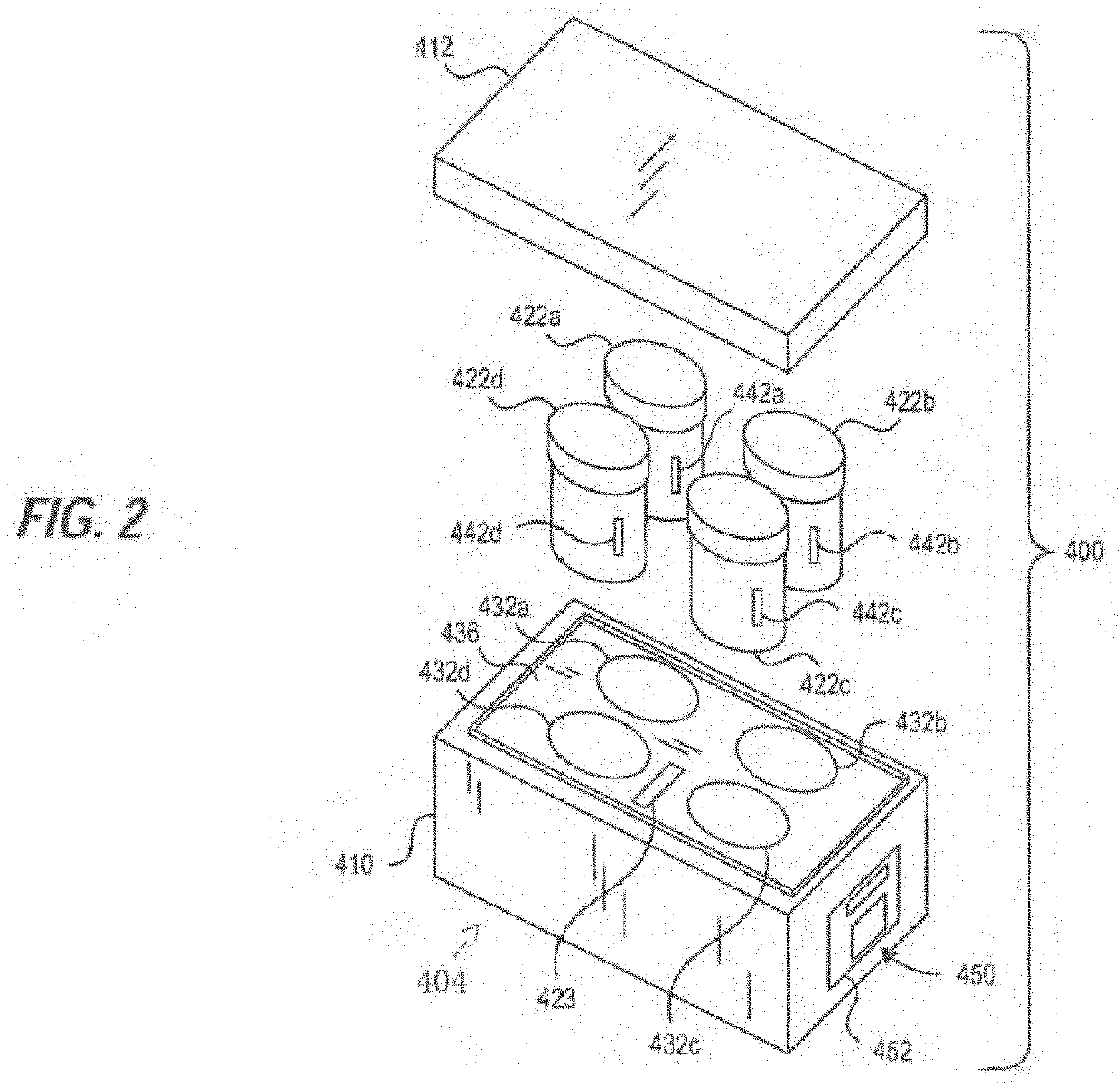
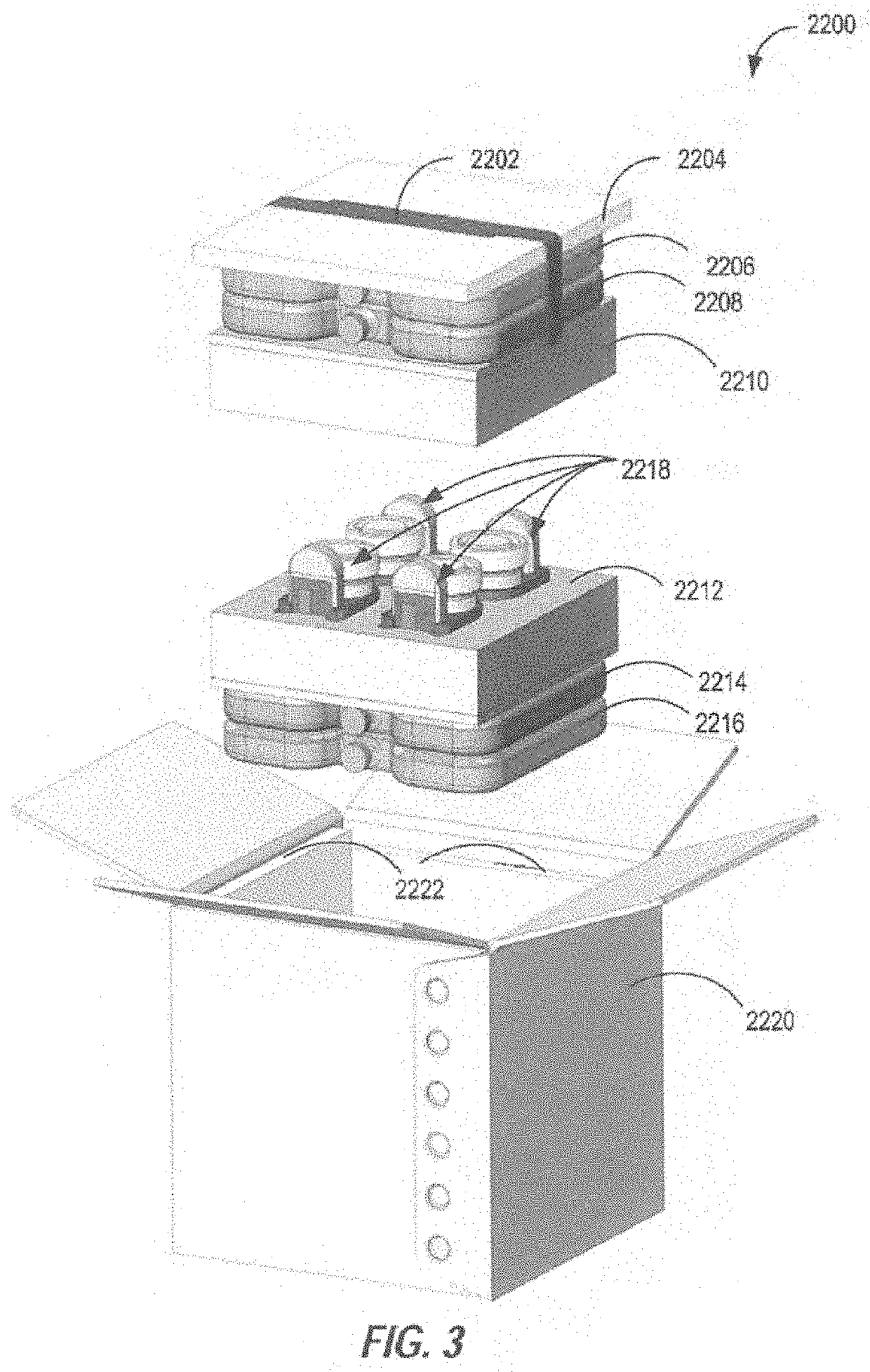
Transporter systems, assemblies and associated methods for transporting tissue samples
a technology of tissue samples and assemblies, applied in the field of transporter systems, assemblies and associated methods for transporting tissue samples, can solve the problems of poor tissue morphology, under or over exposure of tissue fixatives, shrinking cellular structures, and condensing nuclei, so as to facilitate the reading of at least one code
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
[0515]2. The transporter system of embodiment 1 wherein the reagent is a tissue fixation reagent.
[0516]3. The transporter system of embodiment 1 further comprising a tissue sample cassette for retaining one or more tissue samples therein and wherein the specimen container is configured to retain and hold the tissue sample cassette in the reagent.
[0517]4. The transporter system of embodiment 1, wherein the monitoring system measures the temperature of the reagent and / or tissue sample in the specimen container over a period of time.
[0518]5. The transporter system of embodiment 1, further including an insulator and / or a cooling element capable of maintaining the volume of the formalin-based fixative solution at a temperature in the range of 0° C. to 15° C. for at least 2 hours under shipping conditions when the tissue sample transport and storage assembly is assembled.
[0519]6. The transporter system of embodiment 1, wherein the monitoring system is physically coupled to the carrier ass...
embodiment 4
[0523]10. The transporter system of embodiment 4, wherein the specimen container comprises a temperature sensor wherein said sensor transmits the temperature of the reagent contained therein to the monitoring system.
[0524]11. The transport system of embodiment 1, wherein the specimen container further comprises a radio frequency identification (RFID) tag and the monitoring system further comprises a RFID reader.
[0525]12. The transport system of embodiment 1, further comprising a transport container for transporting the specimen container, carrier assembly and monitoring system wherein the transport container comprises an ice bath.
[0526]13. The transport system of embodiment 1, further comprising a transport container for transporting the specimen container, carrier assembly and monitoring system, wherein the transport container is configured to maintain the temperature of a specimen container therein to from about 0° C. to about 15° C. for up to 14 days.
[0527]14. The transporter sys...
embodiment 16
[0534]17. The apparatus of embodiment 16, wherein the sensor assembly of each of the plurality of carrier assemblies comprises a temperature sensor positioned within the specimen container, wherein the temperature sensor includes a transmitter that outputs a temperature signal to a receiver located in the carrier assembly holding that specimen container.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com