Methods for treating neuroblastoma
a neuroblastoma and treatment method technology, applied in the field of cancer biology and medicine, can solve the problem that the overall long-term survival of patients with high-risk diseases remains poor at approximately 50%, and achieve the effects of reducing the risk of allodynia, and reducing the overall polyamine conten
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Oral DFMO and Etoposide
[0218]The primary aim of the phase I clinical trial was to study the safety of the ODC inhibitor α-difluoromethylornithine (DFMO) alone and in combination with a cytotoxic chemotherapeutic drug in pediatric patients with refractory or recurrent NB. Etoposide was chosen for the combination, as it has reported efficacy in this patient group (Kushner et al., 2013) and is synergistic with DFMO in some cell models (Dorr et al., 1986). The secondary aims were to investigate the activity, pharmacokinetics and genetic and metabolic factors associated with ODC.
[0219]No dose-limiting toxicities (DLTs) or drug related serious adverse events (SAEs) were observed in this study. Study related (possibly, probably, and definitely related) toxicities observed during all cycles are summarized in Table 3. Those related to DFMO alone consisted of anemia (N=3), ANC decrease (N=2), decreased platelet count (N=2), ALT increase (N=1), AST increase (N=1), anorexia (N=1), constipation ...
example 2
inetics of DFMO in Children with NB
[0220]DFMO serum measurements were performed in all 21 patients. Samples were collected from patients prior to, and again at times 0.5, 1, 3, and 6 hours following drug administration on days 1 and 8 of the first cycle. DFMO serum samples were also collected from selected patients in the higher dose groups (750, 1000, 1500 mg / m2) during cycle 2. FIG. 4 shows the serum DFMO concentrations (mean and sd) in all patients receiving 750 mg / m2 (mean±standard deviation). DFMO doses were administered orally twice daily over a 21 day cycle. Subsequent cycles commenced the day following the last day of the previous cycle. Maximum DFMO concentrations, relative to dose, are reported in Table 4. Overall average serum DFMO concentrations ranged from 9.54 μg / mL (52.24 μM) in patients receiving 500 mg / m2 to 30.71 μg / mL (168.10 μM) in patients receiving 1500 mg / m2. The mean tmax occurred between 2.50 and 3.75 hours, in all dose groups. The mean AUC0-6 h ranged from ...
example 3
for Genetic and Metabolic Markers of Polyamine Metabolism and Pharmacodynamic (PD) Measures of DFMO Effect
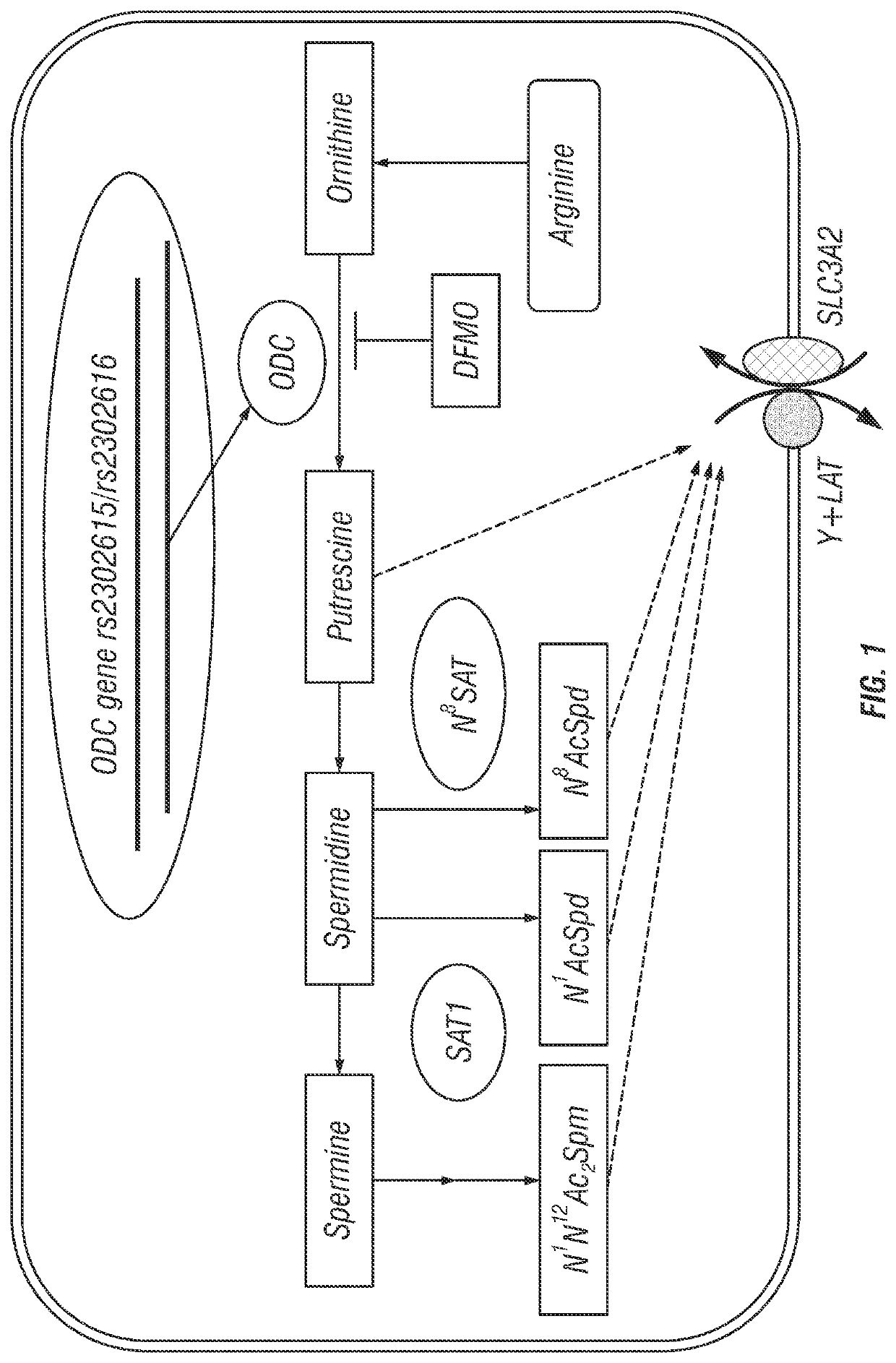
[0222]FIG. 1 depicts the polyamine metabolic pathway and highlights the relationship between ODC genotypes (rs2302615 and rs2302616), affecting ODC expression, and their relationship to urinary polyamines. The figure shows the substrate relationships for the diamine and acetylpolyamine exporter (Xie et al., 1997; Uemura et al., 2008; Uemura et al., 2010), which include putrescine, monoacetylspermidine and diacetylspermine (DAS) but not spermidine or spermine. Levels of these exported amines might be expected to reflect changes in tissue ODC expression, as polyamine export is known as one component of polyamine homeostatic regulation (Gerner and Meyskens, 2004).
[0223]First morning void spot urines from each patient were evaluated for polyamines as described in Methods. Table 5 shows data (means±SD) at baseline (cycle 1, day 1) for seven metabolites in the polyamine pathway, inclu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com