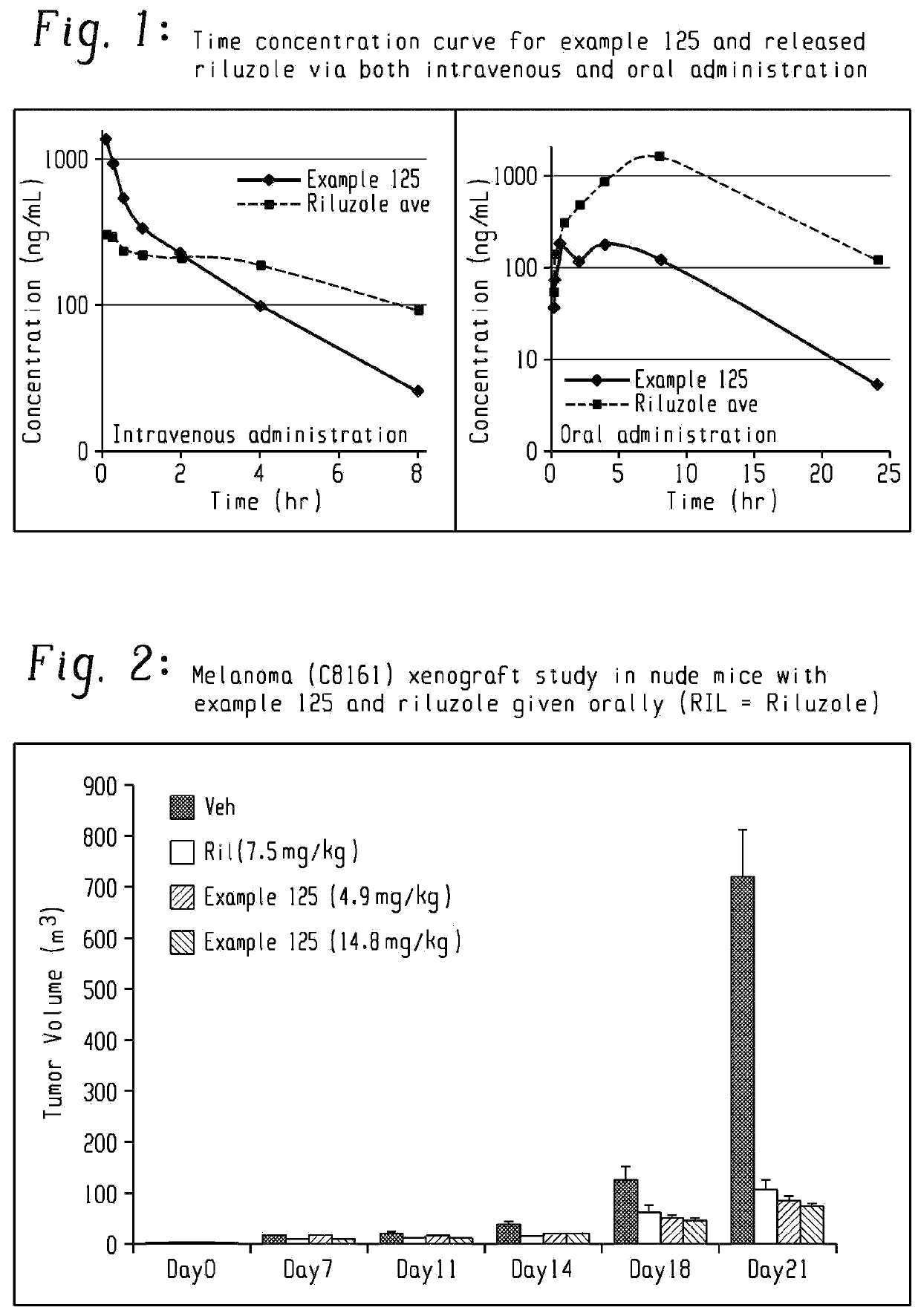
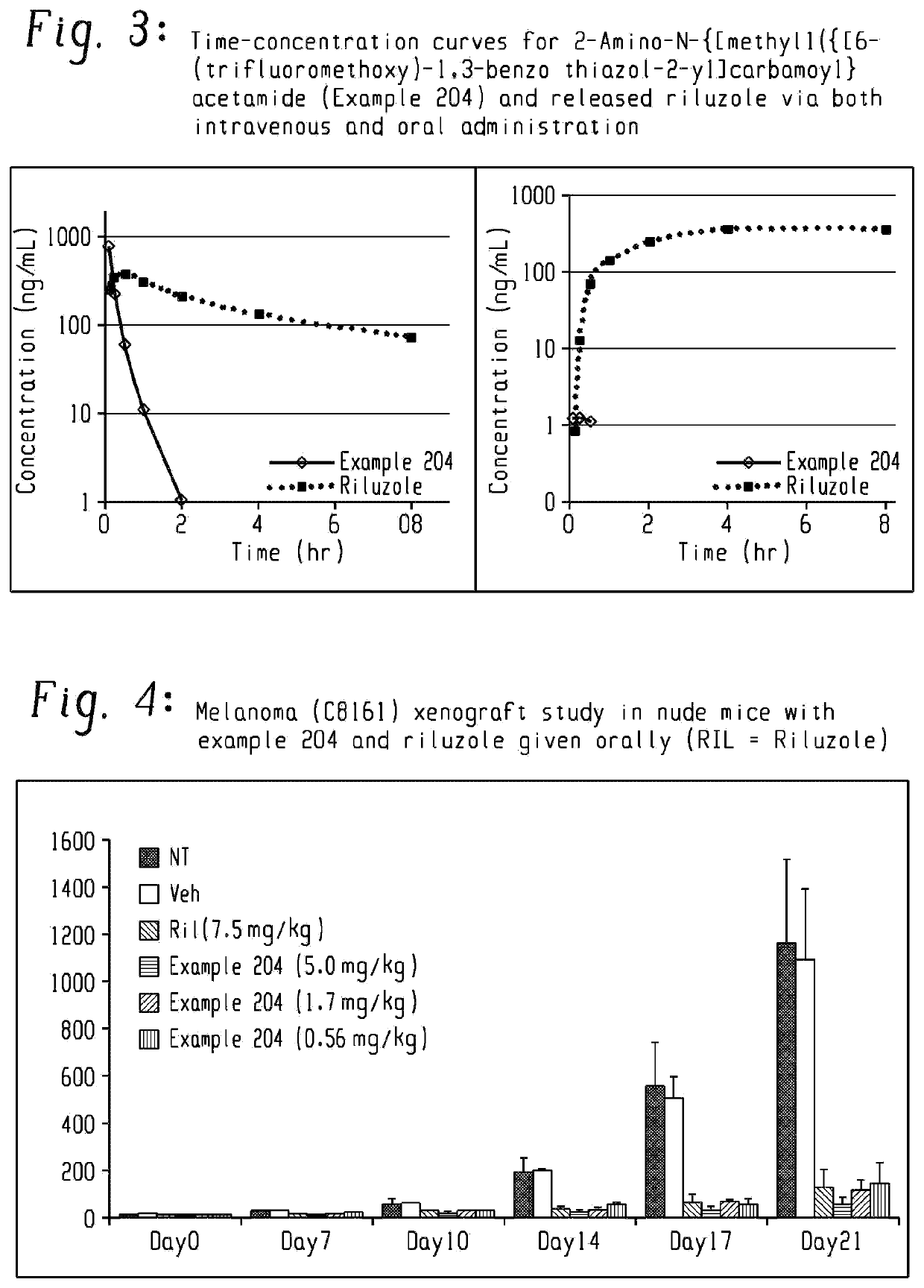
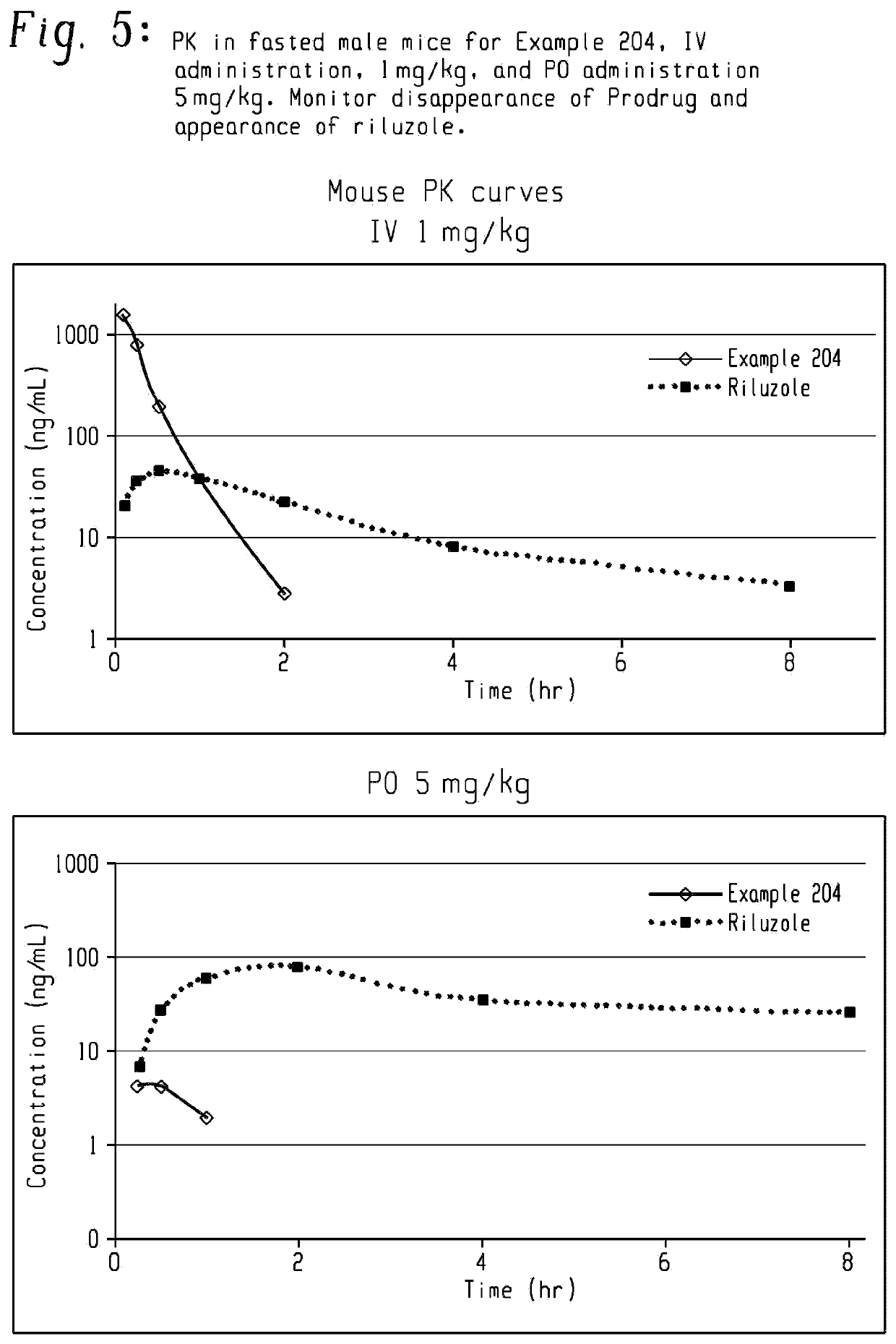
Prodrugs of riluzole and their method of use
a technology of riluzole and prodrugs, which is applied in the field of prodrugs of riluzole and their method of use, can solve the problems of limited clinical application of riluzole to other disease states, low solubility of riluzole in water, and liver metabolism problems, and achieve the effect of enhancing the activity of a serotonin reuptake inhibitor (sri)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of 2-amino-N-(6-(trifluoromethoxy)benzo[d]thiazol-2-yl)acetamide
[0837]
[0838]To a solution of 2-amino-6-(trifluoromethoxy)benzimidazole (0.50 g, 2.1 mmol), N-(t-butyloxycarbonyl) glycine (0.56 g, 3.2 mmol) and N,N-diisopropylethylamine (0.41 g, 3.2 mmol, 0.57 ml) in dimethylformamide (7 ml) was added 1[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU, 1.2 g, 3.2 mmol) and the mixture stirred at 20° C. for 18 hours. Ethyl acetate (100 ml) was added and the mixture was washed with water (2×75 ml), 1N HCl (75 ml), water (75 ml), 1M sodium carbonate solution (75 ml) and brine. The organic layer was dried over sodium sulfate and evaporated. The residue was chromatographed on silica gel eluted with a gradient of ethyl acetate in hexanes (50% to 75%) to leave the product as a white foamy solid (0.78 g, 95%). LC / MS method A: Rt=5.92 min., (M+H)+=392. The product was dissolved in 4N HCl / 1,4-dioxane and stirred for 2 h. The solvents were evaporat...
example 2
of 2-(Methylamino)-N-(6-(trifluoromethoxy)benzo[d]thiazol-2-yl) acetamide hydrochloride
[0839]
[0840]2-(Methylamino)-N-(6-(trifluoromethoxy)benzo[d]thiazol-2-yl) acetamide hydrochloride was prepared according to the procedure of example 1 from 2-amino-6-(trifluoromethoxy) benzimidazole (0.50 g, 2.1 mmol) and N-(t-butyloxycarbonyl)sarcosine (0.60 g, 3.2 mmol). Yield for intermediate=0.96 g (100%). LC / MS method A: Rt=5.92 min., (M+H)+=406. Yield for final product (0.82 g, 100%, 100% overall). LC / MS method A: Rt=3.60 min., (M+H)+=306.
example 3
of 2-(Ethylamino)-N-(6-(trifluoromethoxy)benzo[d]thiazol-2-yl)acetamide hydrochloride
[0841]
[0842]2-(Ethylamino)-N-(6-(trifluoromethoxy)benzo[d]thiazol-2-yl)acetamide hydrochloride was prepared according to the procedure of example 1 from 2-amino-6-(trifluoromethoxy)benzimidazole (117 mg, 0.5 mmol) and N-t-butyloxycarbonyl-2-(ethylamino)acetic acid (122 mg, 0.6 mmol). Yield for Boc protected intermediate 182 mg (87%). LC / MS method A: Rt=6.20 min., (M+H)+=420. Yield for final product (142 mg, 87% overall). LC / MS method A: Rt=3.67 min., (M+H)+=320.
PUM
| Property | Measurement | Unit |
|---|---|---|
| survival time | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com