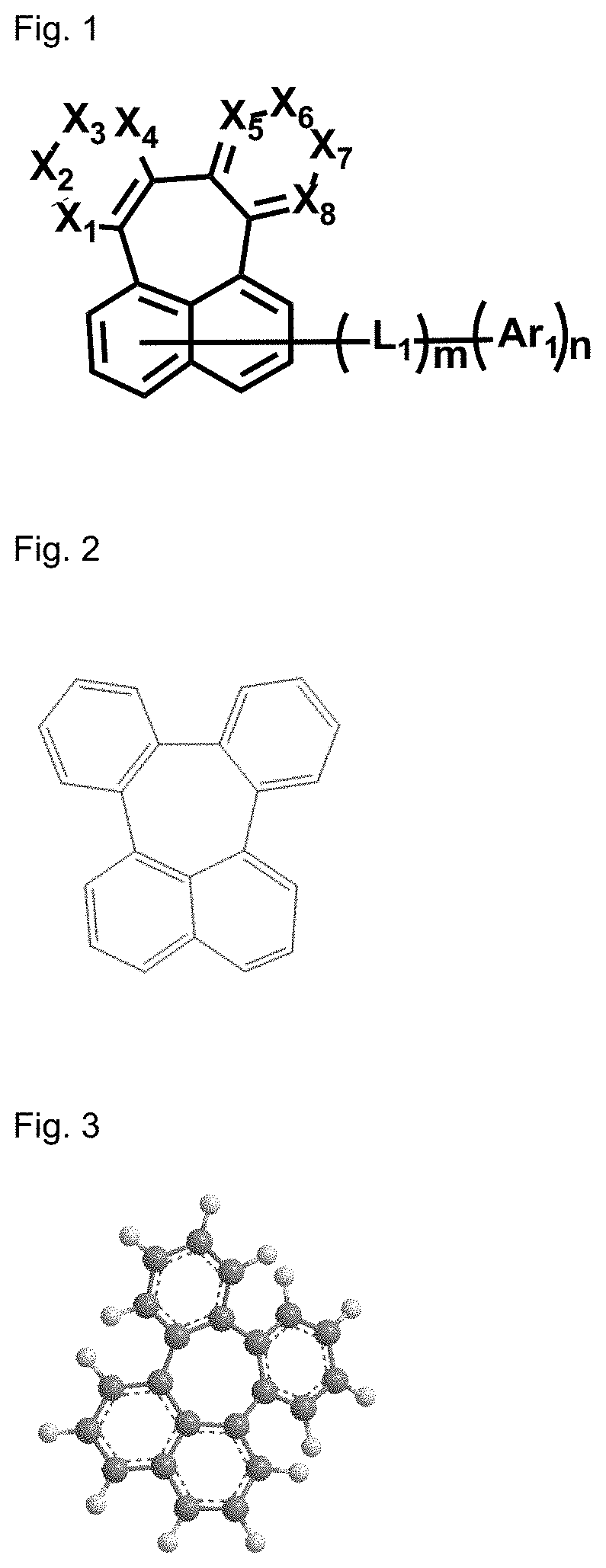
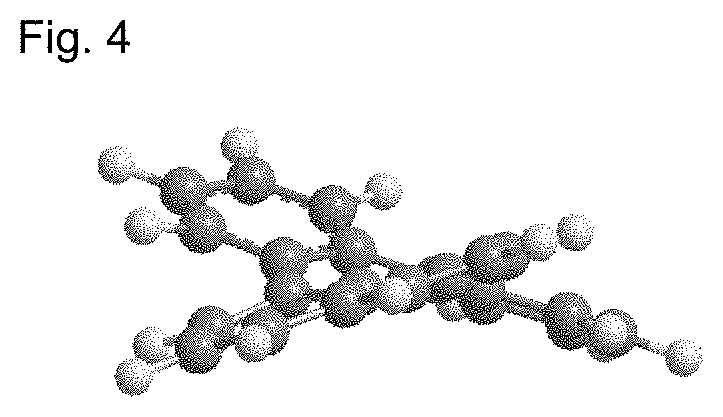
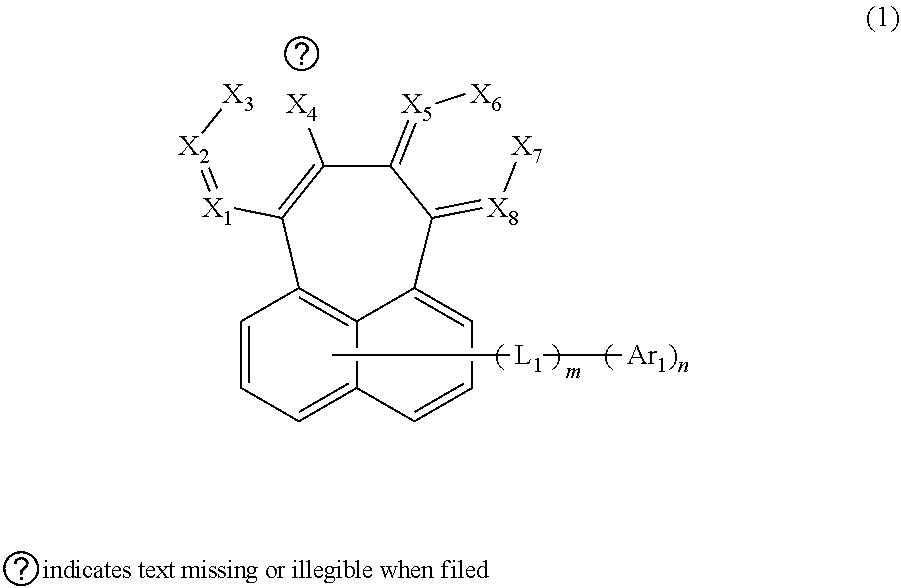
Organic electroluminescent compound and organic electroluminescent device comprising the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
on of Compound A-22
[0083]
[0084]1) Preparation of Compound 1-5
[0085]7.0 g of compound 1-4 (19.6 mmol), 4.0 g of 4-chlorophenylboronic acid (25.5 mmol), 1.13 g of tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) (0.98 mmol), and 6.8 g of potassium carbonate (49 mmol) were dissolved in 100 mL of tetrahydrofuran and 25 mL of distilled water in a flask, and the mixture was refluxed at 100° C. for 18 hours. After completion of the reaction, an organic layer was extracted with ethyl acetate, the remaining moisture was removed using magnesium sulfate, and the residue was dried and separated with column chromatography to obtain 6.1 g of compound 1-5 (yield: 80%)
[0086]2) Preparation of Compound A-22
[0087]6.1 g of compound 1-5 (15.7 mmol), 6.3 g of N-1,1′-biphenyl-4-yl-9,9-dimethyl-9H-fluorene-2-amine (17.3 mmol), 0.72 g of tris(dibenzylideneaceton)dipalladium(0) (0.80 mmol), 0.64 mL of tri-t-butylphosphine (1.57 mmol, 50% toluene solution), 3.0 g of sodium t-butoxide (31.4 mmol), and 160 ...
example 3
on of Compound A-13
[0088]
[0089]1) Preparation of Compound 1-7
[0090]15 g of compound 1-1 (47.2 mmol), 25 g of compound 1-6 (47.2 mmol), 2.7 g of tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) (2.36 mmol), and 16.3 g of potassium carbonate (118 mmol) were dissolved in 240 mL of tetrahydrofuran and 60 mL of distilled water in a flask, and the mixture was refluxed at 100° C. for 18 hours. After completion of the reaction, an organic layer was extracted with ethyl acetate, the remaining moisture was removed using magnesium sulfate, and the residue was dried and separated with column chromatography to obtain 14.8 g of compound 1-7 (yield: 49%)
[0091]2) Preparation of Compound A-13
[0092]14 g of compound 1-7 (22.1 mmol), 0.99 g of Pd(OAc)2 (4.42 mmol), 2.44 g of ligand(tricyclohexylphosphonium tetrafluoroborate) (6.63 mmol), 21.6 g of Cs2CO3 (66.3 mmol), and 110 mL of dimethyl acetamide (DMA) were stirred under reflux for 3 hours. The mixture was cooled to room temperature and distille...
example 1
Device Production of an OLED Using the Organic Electroluminescent Compound According to the Present Disclosure
[0094]An OLED using the organic electroluminescent compound according to the present disclosure was produced as follows. A transparent electrode indium tin oxide (ITO) thin film (10 Ω / sq) on a glass substrate for an OLED (Geomatec, Japan) was subjected to an ultrasonic washing with acetone and isopropanol, sequentially, and was then stored in isopropanol. Next, the ITO substrate was mounted on a substrate holder of a vacuum vapor depositing apparatus. Compound HIL-1 was introduced into a cell of said vacuum vapor depositing apparatus, and then the pressure in the chamber of said apparatus was controlled to 10−6 torr. Thereafter, an electric current was applied to the cell to evaporate the above-introduced material, thereby forming a first hole injection layer having a thickness of 90 nm on the ITO substrate. Compound HIL-2 was then introduced into another cell of said vacuu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Transport properties | aaaaa | aaaaa |
| Electroluminescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com