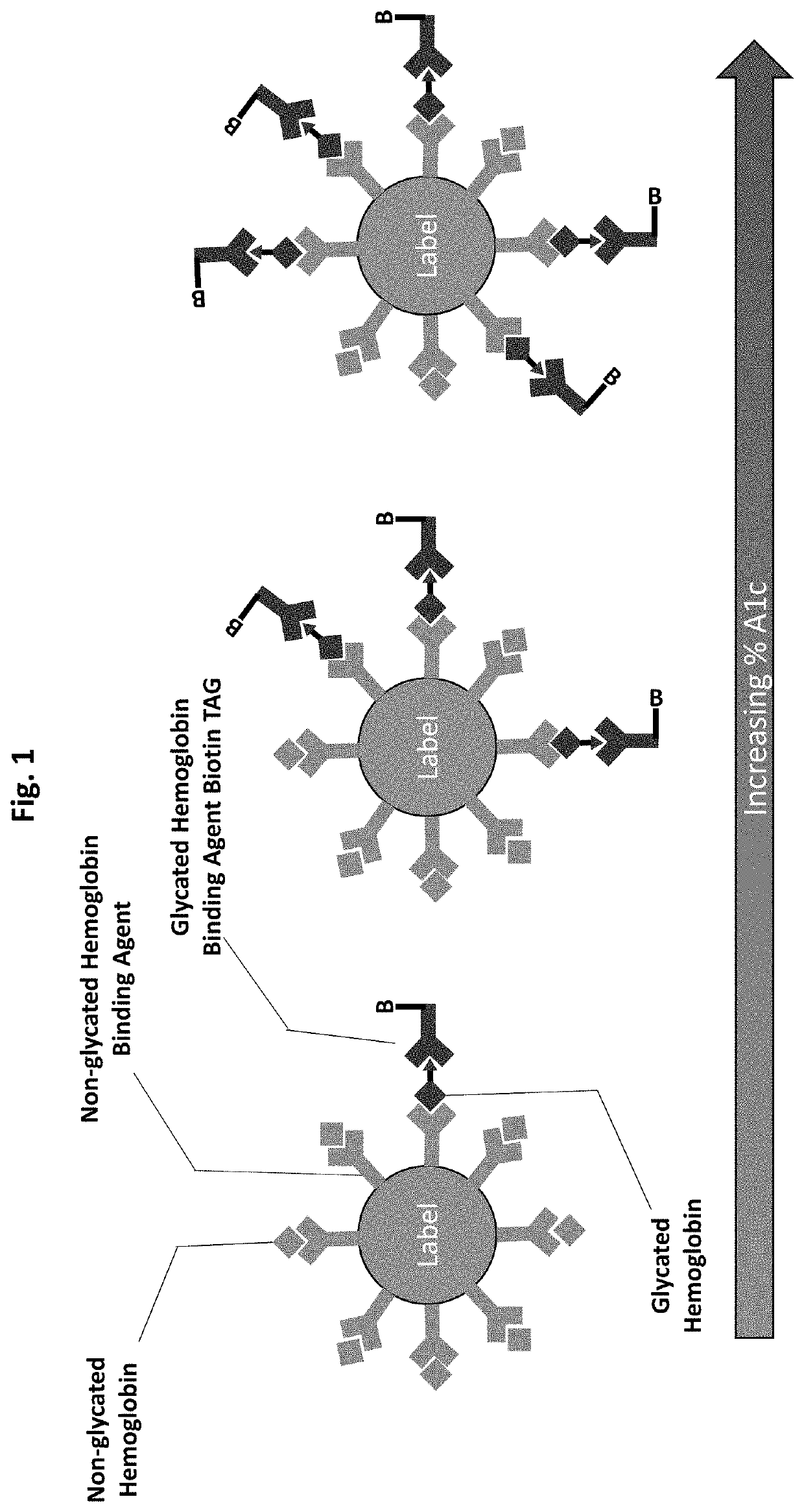
Saturation binding ratiometric assay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
h Magnetic Anti-Hemoglobin Particles
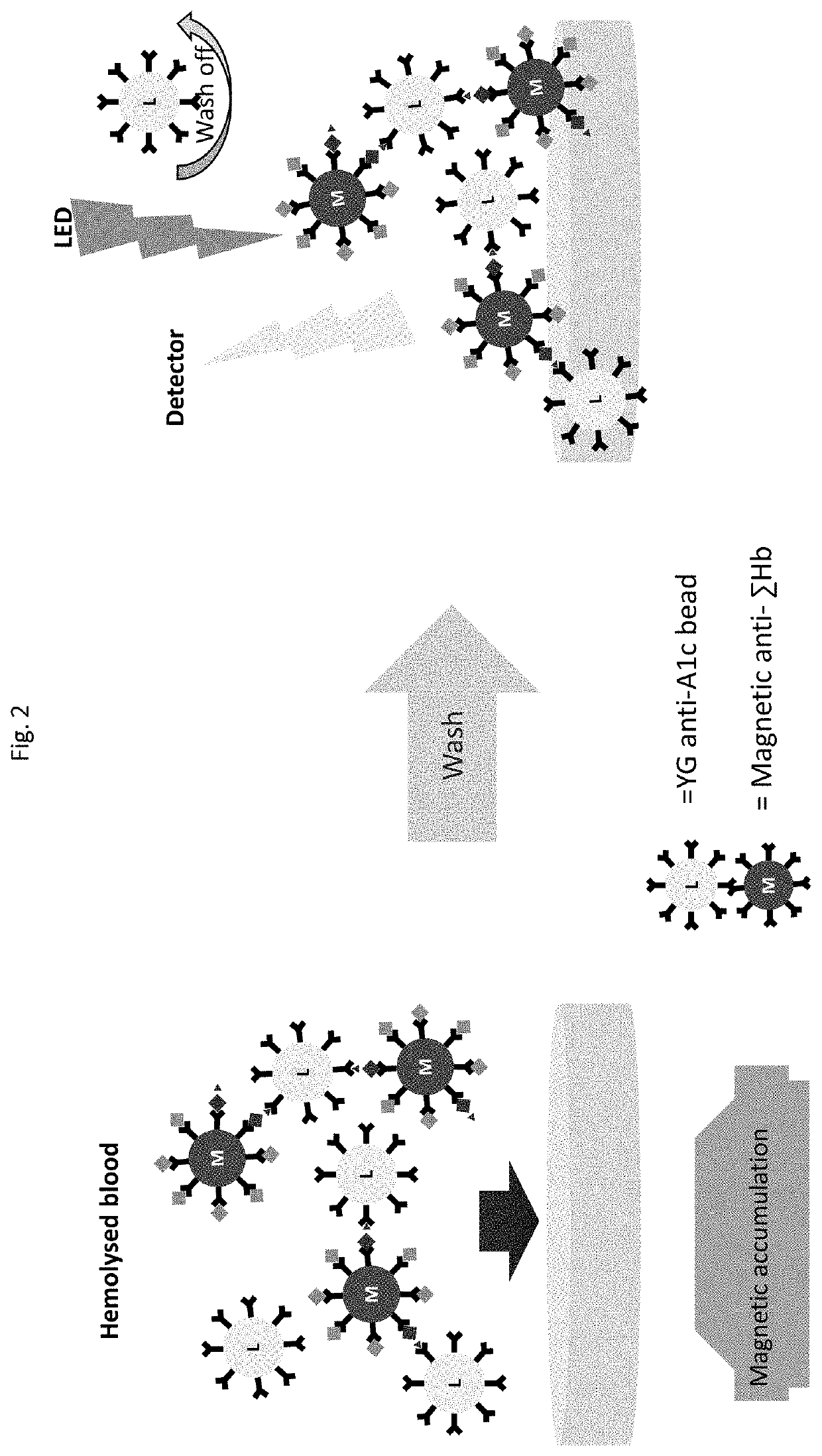
[0135]Assays were carried out using magnetic beads bearing surface anti-total hemoglobin antibodies as schematically shown in FIG. 2. The antibodies were obtained from mice using an immunogen containing hemoglobin beta chain amino terminal peptide and selected as described above, such that the antibodies bind with an epitope within amino acids 7-25 of the hemoglobin beta chain and cause exposure of the beta chain amino terminus. The magnetic beads used were 200 nm. The assays were carried out as dry A1c assays, meaning reagents were provided in dry form (as fast reconstituting reagent pellets) and were reconstituted in the assay device during the performance of the assay. To carry out the assays, sample was introduced into a first assay device chamber, thereby reconstituting dry anti-Hb magnetic beads within the chamber, and incubated for 60 s, followed by mixing in the same chamber for 90 s. The magnetic beads were then moved to a second chamber ...
example 2
h Magnetic Anti-Hemoglobin Particles and Undiluted Blood Sample
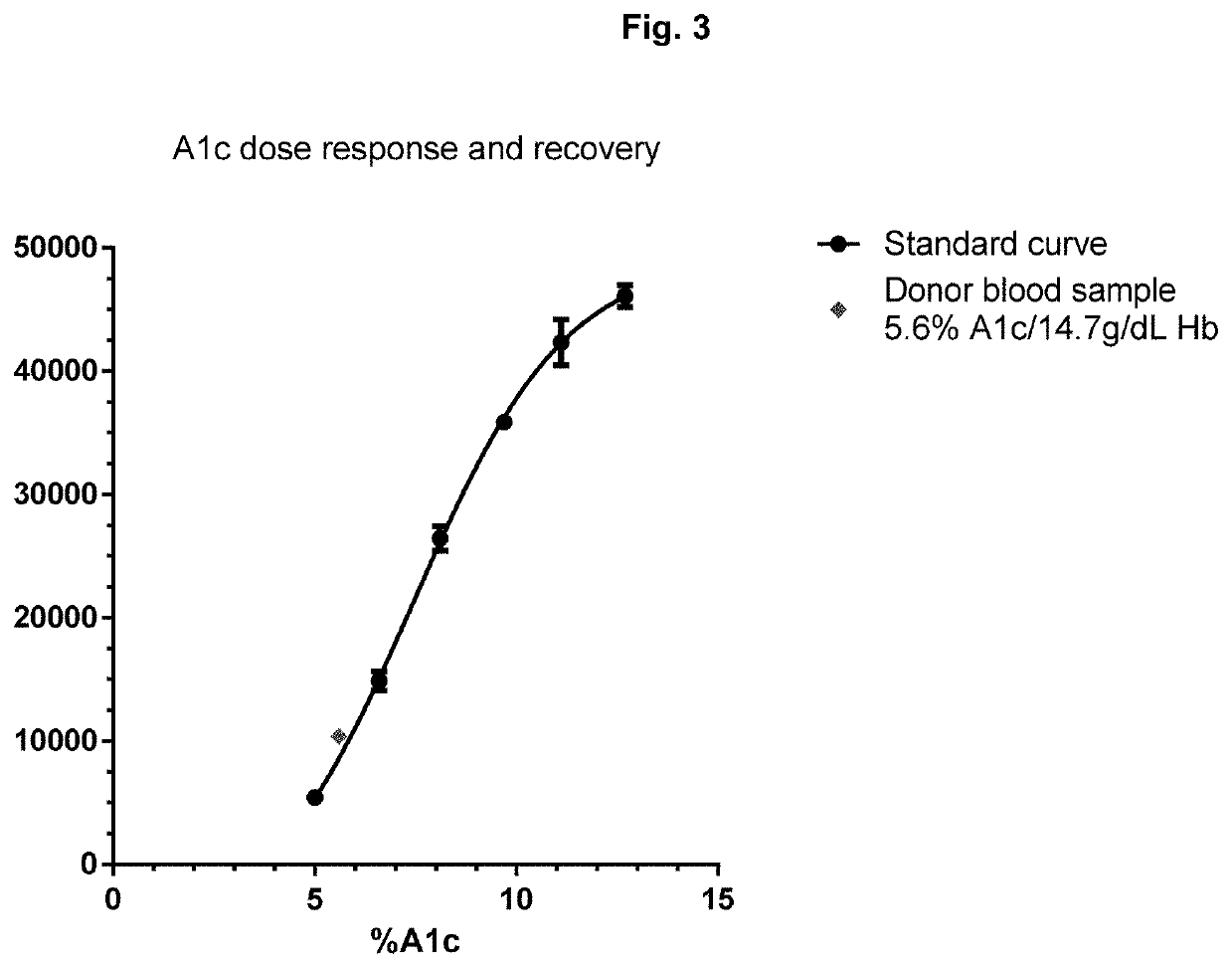
[0141]A magnetic bead assay has been carried out using undiluted blood. For these assays, we used six different undiluted frozen blood samples, which contained 0, 3.9, 6.6, 9.7, 12.7, and 14.4% A1c respectively. The assays were carried out per FIG. 2, wherein magnetic beads modified with anti-total hemoglobin antibody were mixed with hemolyzed whole blood at 14 g / dL hemoglobin. After 5 min incubation, the excess blood was removed from magnetic beads by magnetic separation and the beads were washed 2 more times with a buffer. Time resolved fluorescent (TRF) beads modified with anti-A1c antibody were added to the washed magnetic beads. After 5 min incubation the unbound TRF beads were washed thrice with buffer with an aid of a magnet. The TRF signal from magnetic-TRF sandwich immunocomplexes was recorded in a TRF reader. The results shown in FIG. 7 demonstrate that the present assays can be effectively carried out using un...
example 3
low Hb A1c Assay
[0142]Another illustrative assay has been carried out using TRF label in lateral flow configuration.
[0143]In lateral flow assay format, 5 uL of hemolyzed A1c calibrators were mixed with 3 uL of 100 ug / mL time resolved fluorescent (TRF) beads modified with anti-total hemoglobin antibody. After 5 min incubation at room temperature (rt), 2 uL of 35 ug / mL biotinylated A1c antibody was added followed by additional 5 min incubation at room temperature (rt). The reaction mixture was then allowed to run by capillary forces up a nitrocellulose membrane strip containing test streptavidin zone and a reference anti-mouse antibody striped zone. The reference zone is designed to capture any excess of unbound beads modified with mouse anti-total hemoglobin antibody. The reaction mixture was finally chased with an elution buffer to clear unbound particles. The finished and dried strips were read in a TRF reader to provide results shown in FIG. 8. Dose responses from both test and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com