Coenzyme Q10 Aerosol
a technology of coenzyme q10 and aerosol, which is applied in the direction of dispersed delivery, anti-noxious agents, drug compositions, etc., can solve the problems of inability to mill, difficulty in formulating, etc., and achieves high coq10 concentration, high and sustained systemic concentration, and high lipophilic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0058]Method
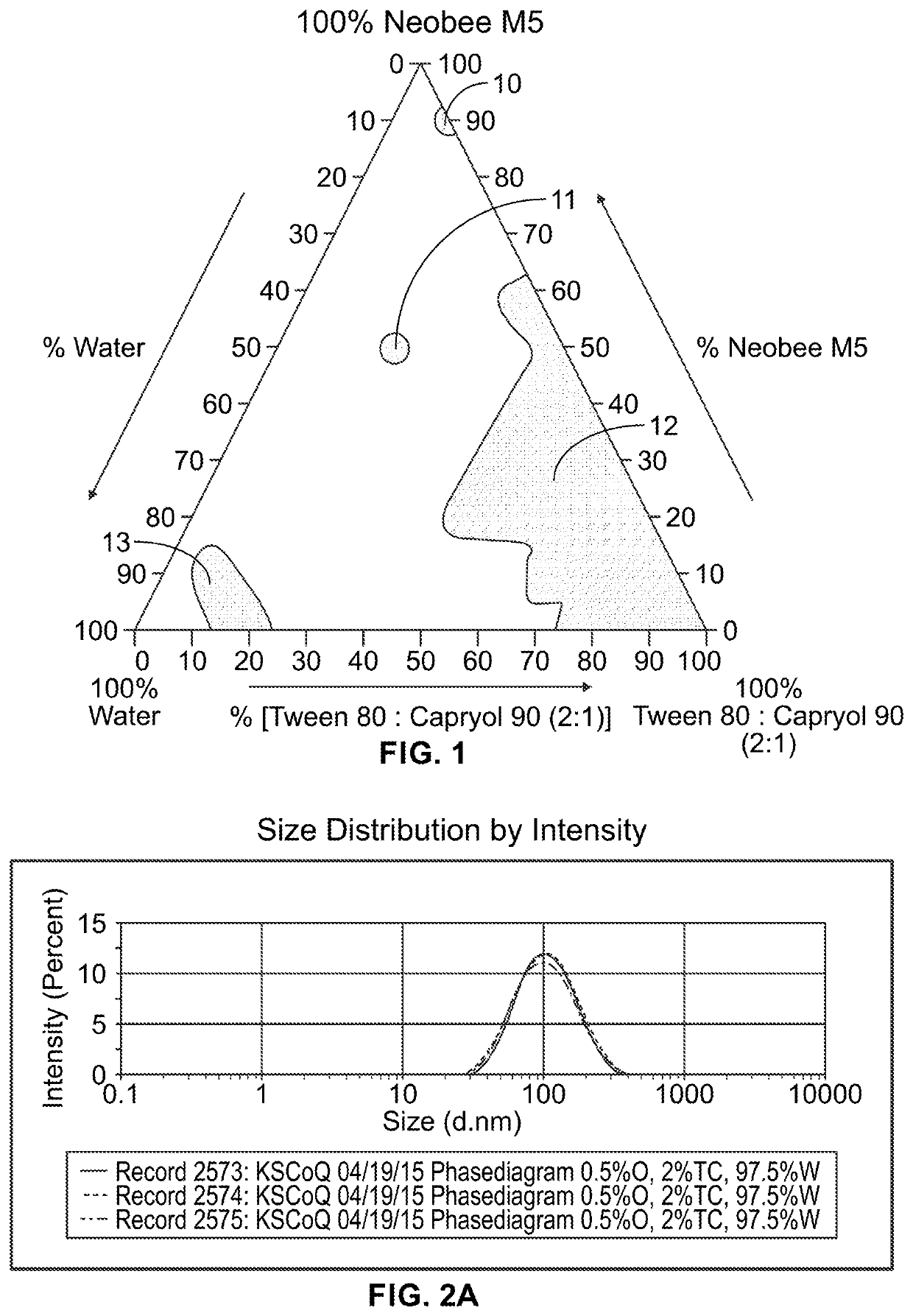
[0059]A mixture of surfactants (e.g., Tween80 and Capryo190) is made and vortexed for about 2 minutes.
[0060]The triglyceride of coconut oil was weighed in a glass vessel. It was then allowed to stand for one hour in a water bath with 37° C. along with the CoQ10, after which it was cooled down and the surfactant mixture was added. This composition was then mixed with the vortexer for about 30 seconds. After which, the composition was allowed to stand until the next day. Water was then added and it was mixed for about 1 min.
[0061]In yet another embodiment, the present provides a method for making and administering an aerosol formulation containing a desired amount of CoQ10 that is particularly useful for creating aerosol treatments having respirable compositions. In this embodiment, the emulsion is isotonic and may be made from an oil in water mixture as well as other isotonic mixtures. In a preferred embodiment, an isotonic agent is used such as dextrose, glycerin, potass...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersion size | aaaaa | aaaaa |
| droplet size | aaaaa | aaaaa |
| droplet size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com