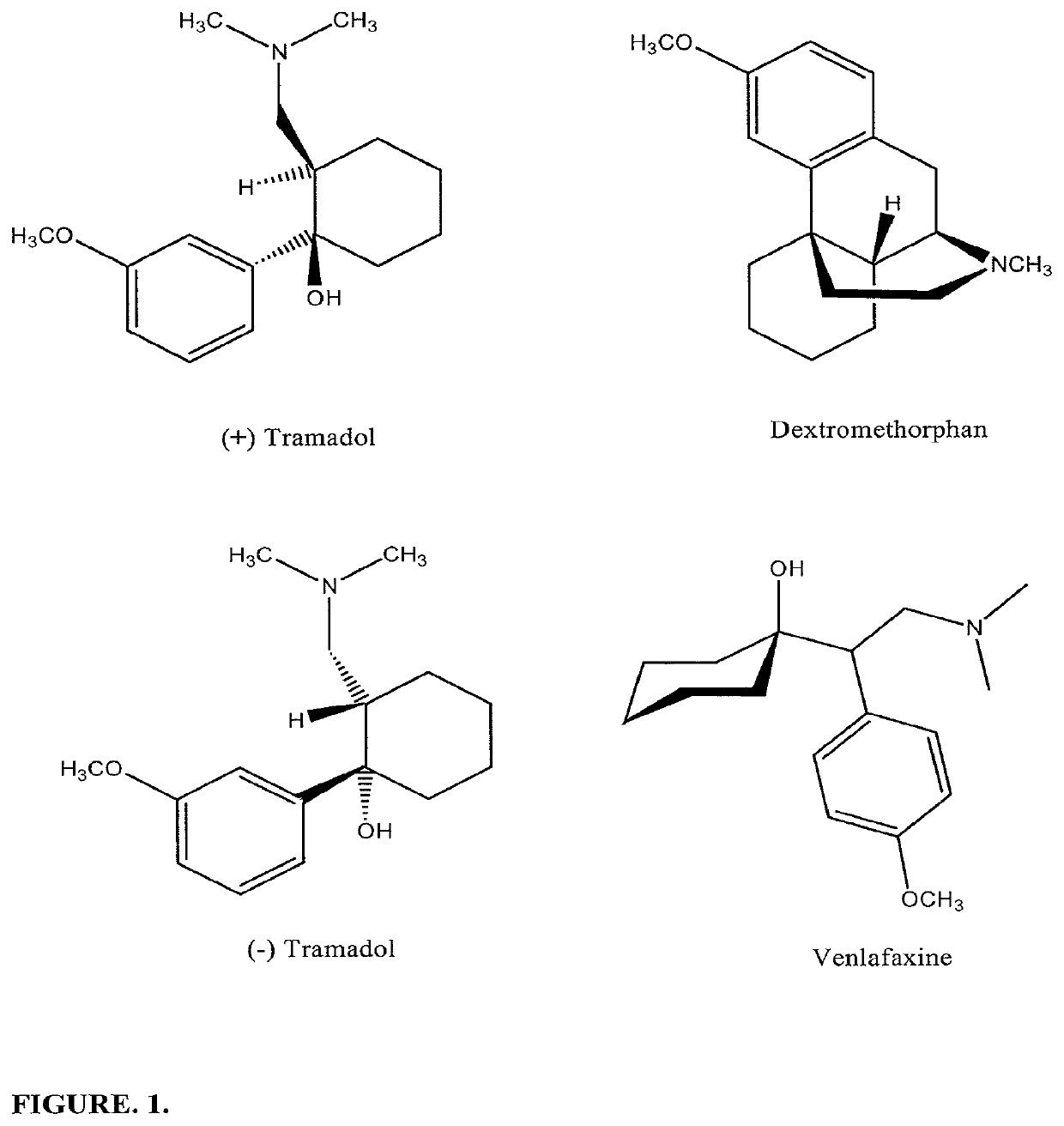
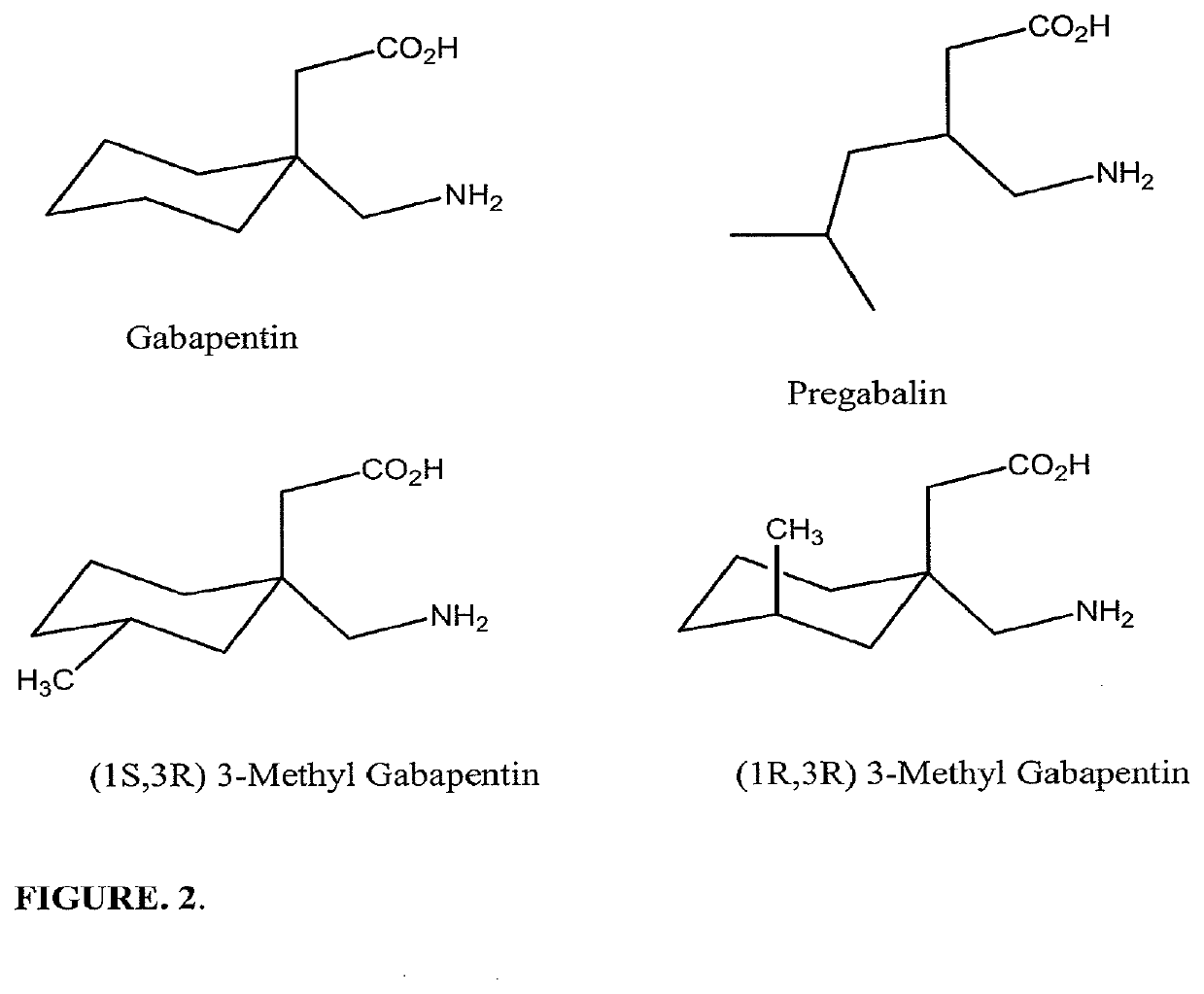
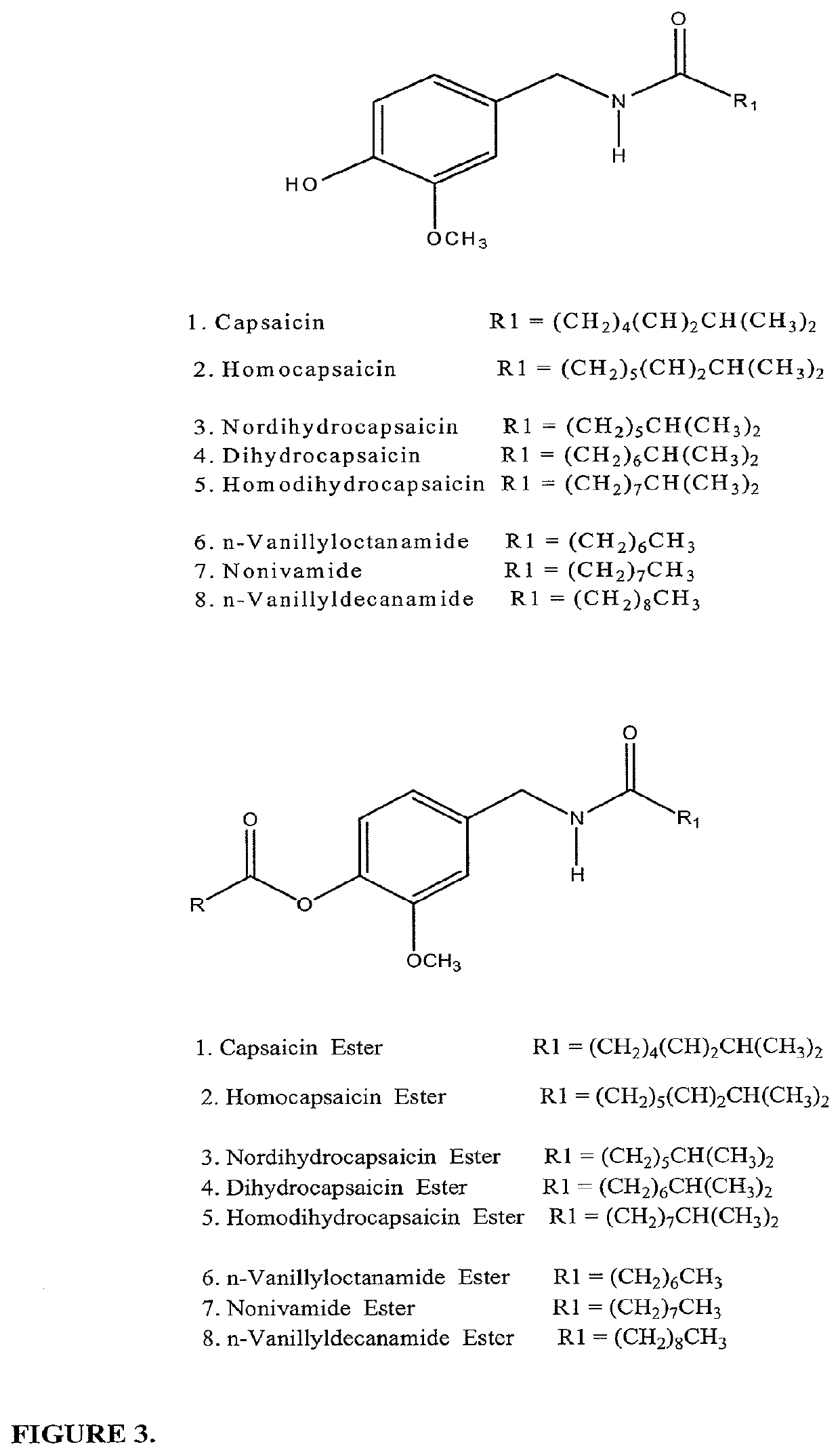
Pharamaceutical compositions for treating chronic pain and pain associated with neuropathy
a technology of pharamaceuticals and compositions, applied in the direction of drug compositions, peptide/protein ingredients, nervous disorders, etc., can solve the problems of not being able to fully understand the pathophysiology of chronic pain, not being able to eliminate having little or no toxicity and/or harmful side effects. , to achieve the effect of avoiding side effects, and eliminating the majority of pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Capsule Formulation Containing Gabapentin
[0280]The following ingredients in each one of the capsule formulations were weighed accurately, ground using a pestle and mortar to fine and homogeneous powders. These powders were sieved through 100 mesh and filled into hard gelatin capsules. The composition of each capsule formulation is listed below.
CAPSULE FORMULATION 1In eachIn 100Tramadol Hydrochloride39.8mg3.98gDextromethorphan51.0mg5.10gHydrochlorideGabapentin90.0mg9.00gMicrocrystalline Cellulose27.6mg2.76gSodium Lauryl Sulfate1.6mg0.16gTotal Solid210mg21.0g
CAPSULE FORMULATION 2In eachIn 100Tramadol Hydrochloride39.8mg3.98gDextromethorphan51.0mg5.10gHydrochlorideGabapentin180.0mg18.00gMicrocrystalline Cellulose77.4mg7.74gSodium Lauryl Sulfate1.6mg0.16gTotal Solid350mg35.0g
CAPSULE FORMULATION 3In eachIn 100Tramadol Hydrochloride35.0mg3.50gDextromethorphan45.0mg4.50gHydrochlorideGabapentin90.0mg9.00gMicrocrystalline Cellulose36.5mg3.65gSodium Lauryl Sulfate2.0mg0.20gTalc1.5mg0.15gTotal...
example 2
Capsule Formulation Containing Pregabalin
[0281]The following ingredients in each one of the capsule formulations were weighed accurately, ground using a pestle and mortar to fine and homogeneous powders. These powders were sieved through 100 mesh and filled into hard gelatin capsules. The composition of each capsule formulation is listed below.
CAPSULE FORMULATION 1In eachIn 100Tramadol Hydrochloride39.8mg3.98gDextromethorphan51.0mg5.10gHydrochloridePregabalin15.0mg1.50gMicrocrystalline Cellulose102.6mg10.26gSodium Lauryl Sulfate1.6mg0.16gTotal Solid210mg21.0g
CAPSULE FORMULATION 2In eachIn 100Tramadol Hydrochloride39.8mg3.98gDextromethorphan51.0mg5.10gHydrochloridePregabalin30.0mg3.00gMicrocrystalline Cellulose87.6mg8.76gSodium Lauryl Sulfate1.6mg0.16gTotal Solid210mg21.0g
CAPSULE FORMULATION 3In eachIn 100Tramadol Hydrochloride35.0mg3.50gDextromethorphan45.0mg4.50gHydrochloridePregabalin20.0mg2.00gMicrocrystalline Cellulose106.5mg10.65gSodium Lauryl Sulfate2.0mg0.20gTalc1.5mg0.15gTot...
example 3
Capsule Formulation Containing Amitriptyline
[0282]The following ingredients in each one of the capsule formulations were weighed accurately, ground using a pestle and mortar to fine and homogeneous powders. These powders were sieved through 100 mesh and filled into hard gelatin capsules. The composition of each capsule formulation is listed below.
CAPSULE FORMULATION 1In eachIn 100Tramadol Hydrochloride39.8mg3.98gDextromethorphan51.0mg5.10gHydrochlorideAmitriptyline Hydrochloride11.4mg11.40gMicrocrystalline Cellulose106.2mg106.2gSodium Lauryl Sulfate1.6mg0.16gTotal Solid210mg21.0g
CAPSULE FORMULATION 2In eachIn 100Tramadol Hydrochloride39.8mg3.98gDextromethorphan51.0mg5.10gHydrochlorideAmitriptyline Hydrochloride5.7mg5.70gMicrocrystalline Cellulose111.9mg11.19gSodium Lauryl Sulfate1.6mg0.16gTotal Solid210mg21.0g
CAPSLUE FORMULATION 3In eachIn 100Tramadol Hydrochloride35.0mg3.50gDextromethorphan45.0mg4.50gHydrochlorideAmitriptyline Hydrochloride11.4mg1.14gMicrocrystalline Cellulose115.1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmaceutical composition | aaaaa | aaaaa |
| healing time | aaaaa | aaaaa |
| compositions | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com