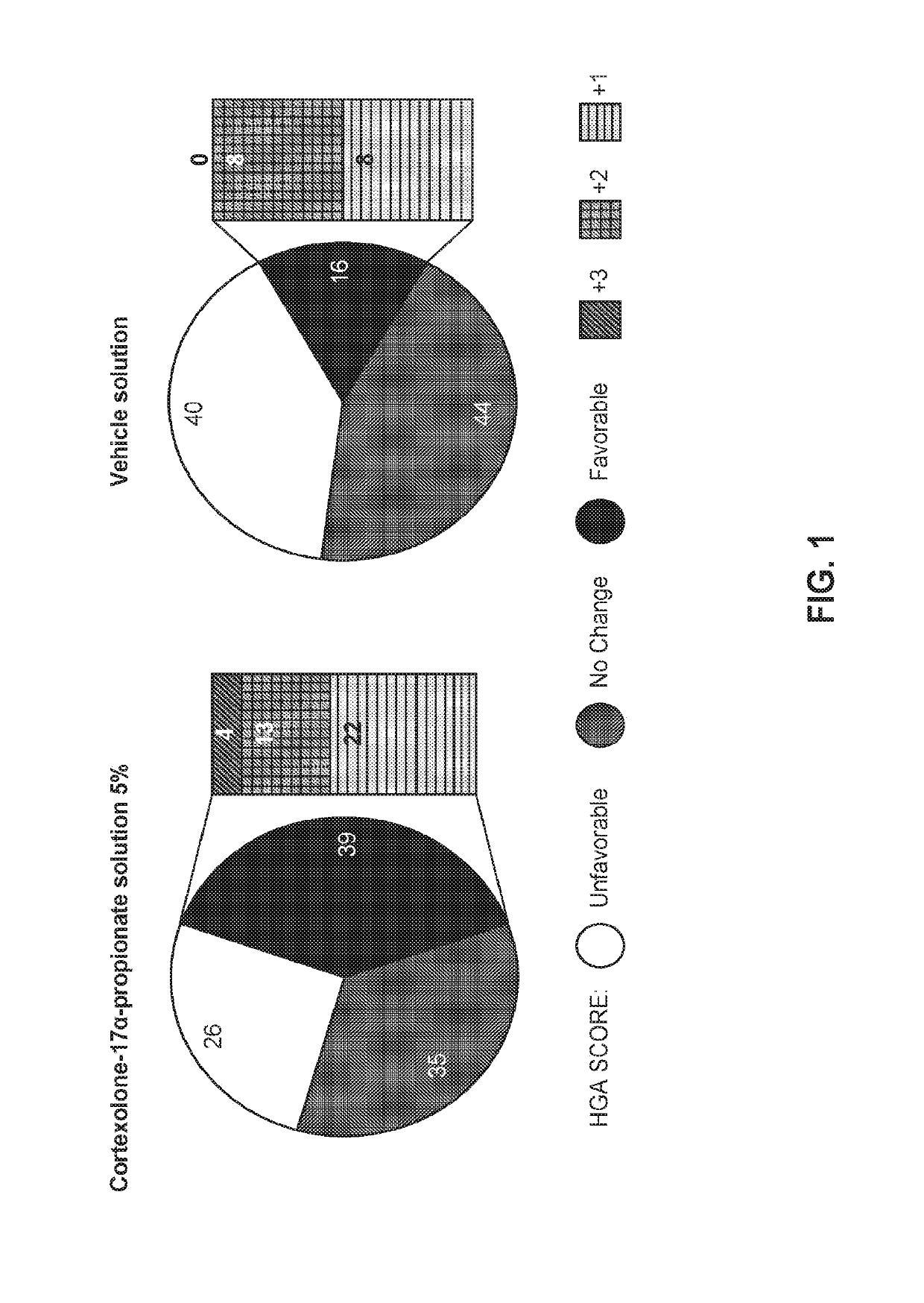
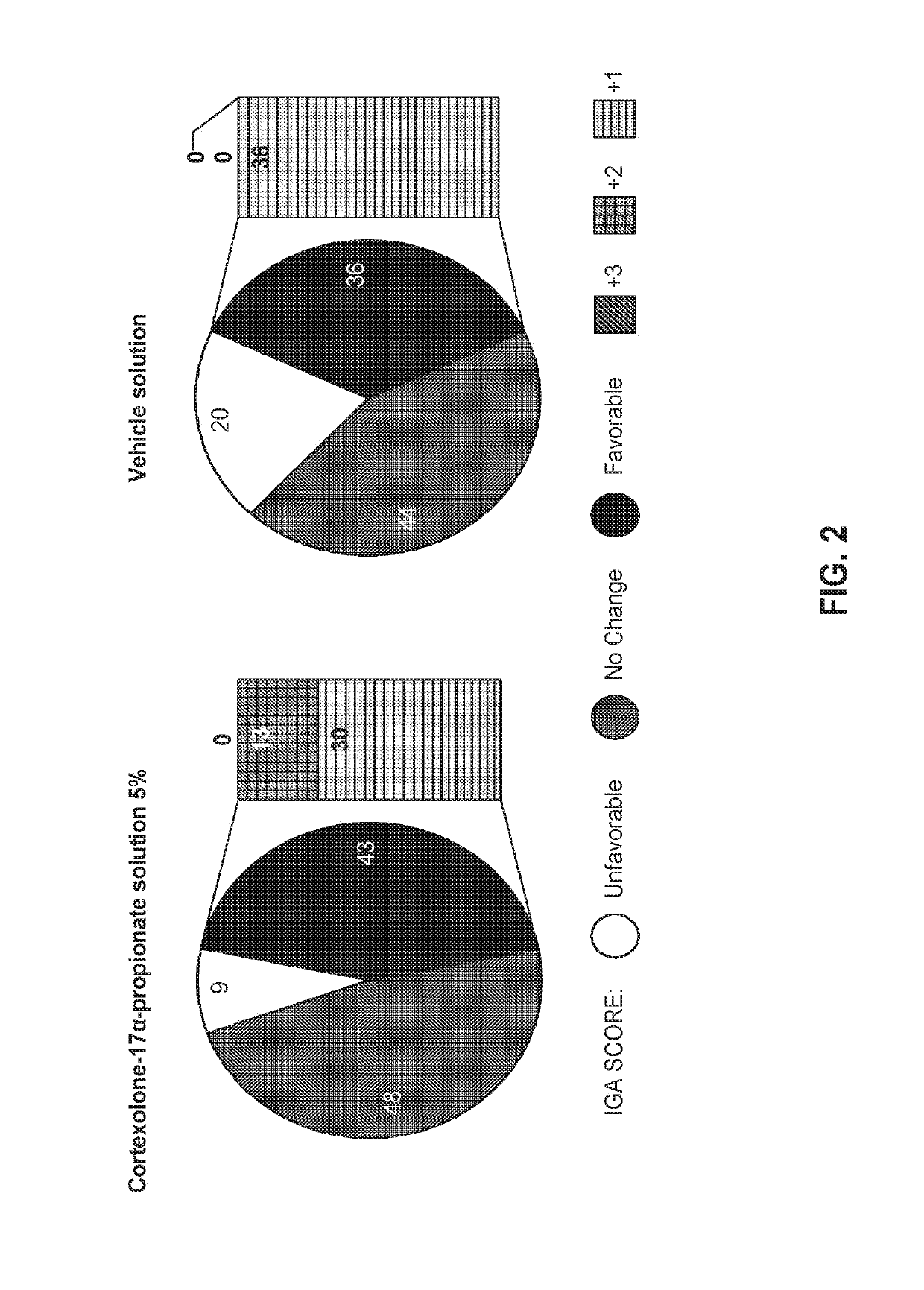
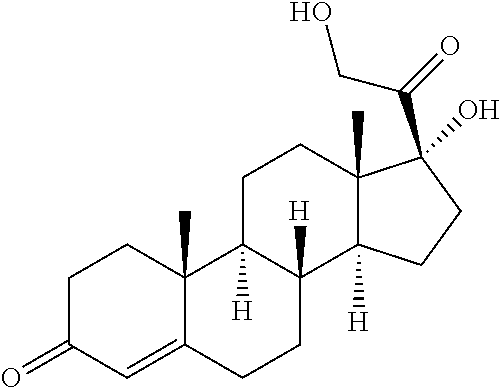
High concentration formulation
a formulation and concentration technology, applied in the field of high concentration formulations, can solve the problems of greater and unwanted systemic exposure to actives and/or their metabolites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evaluation of Cortexolone-17α-propionate Solubility
[0222]5 g of cortexolone-17α-propionate was added to 100 ml of each of the solvents or solvent mixtures shown in Table 1, below, in an effort to produce a 5% w / v solution of cortexolone-17α-propionate. In the examples in Table 1, ethanol refers to ethanol 96°.
TABLE 1Cortexolone-17α-propionate Solubility atSolvent (v:v)Room TemperatureWaterInsolubleEthanolHighly solubleTRANSCUTOL ®Very solublePropylene glycolSparingly solubleIsopropyl myristateInsolubleIsopropyl palmitate,InsolubleCaprylocaproyl polyoxy1-8 glyceridesSparingly solubleNF (LABRASOL ®)TRANSCUTOL ® / water (1:1)InsolubleTRANSCUTOL ® / water / ethanolSoluble(1:1:1)Ethanol / propylene glycol (1:1)SolubleEthanol / propylene glycol / Very solubleTRANSCUTOL ® (1:1:1)Water / propylene glycol / InsolubleTRANSCUTOL ® (1:1:1)Water / propylene glycol / SolubleTRANSCUTOL ® (1:1:2)
[0223]As can be seen from the data in Table 1, water is an unsuitable solvent for cortexolone-17α-propionate when used alone...
example 2
Evaluation of pH on Cortexolone-17α-propionate Stability
[0224]In a suitable container, under stirring, 50 g of cortexolone-17α-propionate was dissolved in a mixture of TRANSCUTOL® (461 g) and ethanol 96° (200 g). After complete dissolution of the cortexolone-17α-propionate, water (283 g) was added slowly. Finally, polysorbate 80 (5 g) and Alpha tocopherols (Vitamin E) (1 g) were added to the formulation. The natural pH of this formulation was measured using a standard pH electrode to be about 5.1.
[0225]The formulation was then split into three equal batches, each weighing about 300 g. Citric acid was added to the first batch to lower the pH to about 4. Sodium citrate was added the second batch to increase the pH to about 6. The third batch, having a natural pH of about 5.1, was used as a control. The three batches were then subjected to a short term stability study at 30° C., with the results shown in Table 2.
TABLE 2Cortexolone-21-propionate Contents*After 1After 2After 3monthmonths...
example 3
Antioxidant Evaluation
[0227]To a formulation containing 5 weight percent cortexolone-17α-propionate in a mixture of TRANSCUTOL®, propylene glycol, and ethanol (96°) in ratio of about 1:1:1 by weight, and further containing polysorbate 80 at 0.1 weight percent, the following antioxidants were added: Alpha tocopherols (Vitamin E) 0.3 weight percent; butylated hydroxyanisole (BHA) 0.01 weight percent, or ascorbyl palmitate 0.5 weight percent. The three formulations were then studied under short term stability conditions. The results are shown in Table 3.
TABLE 3Total Impurities Contents (Impurity percent = (sum of the % (w / w) of each impurity) / % (w / w) of cortexolone 17-alpha propionate * 100)After 1After 2After 3 monthmonthsmonthsAntioxidantTime 0(30° C.)(30° C.)(30° C.)Alpha0.62%1.33%1.56%1.78%tocopherols(Vitamin E)BHA1.37%2.86%2.57%NAAscorbyl0.05%0.22%0.29%0.37%palmitate
[0228]Ascorbyl palmitate provided the least amount of total degradation products.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com