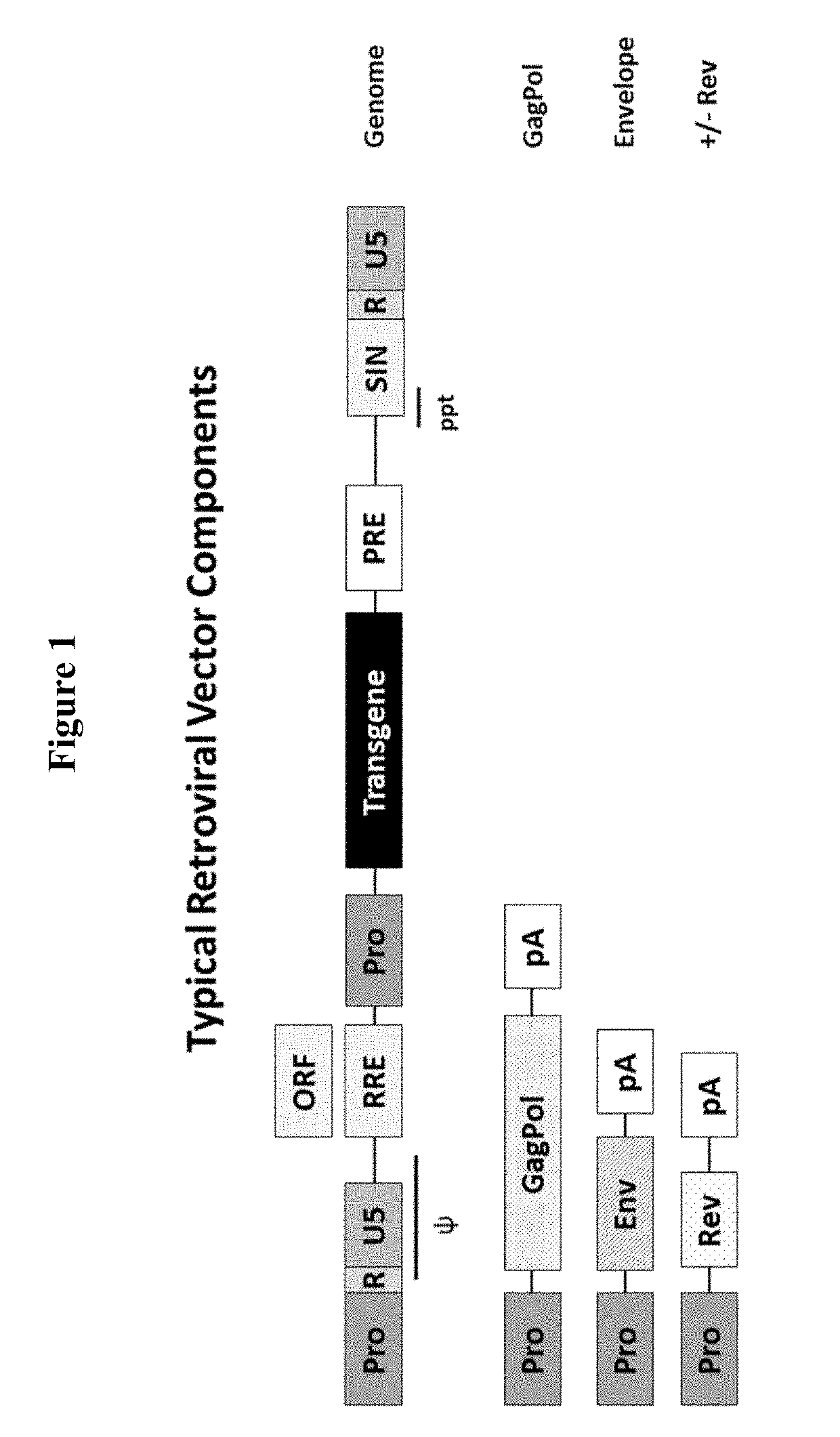
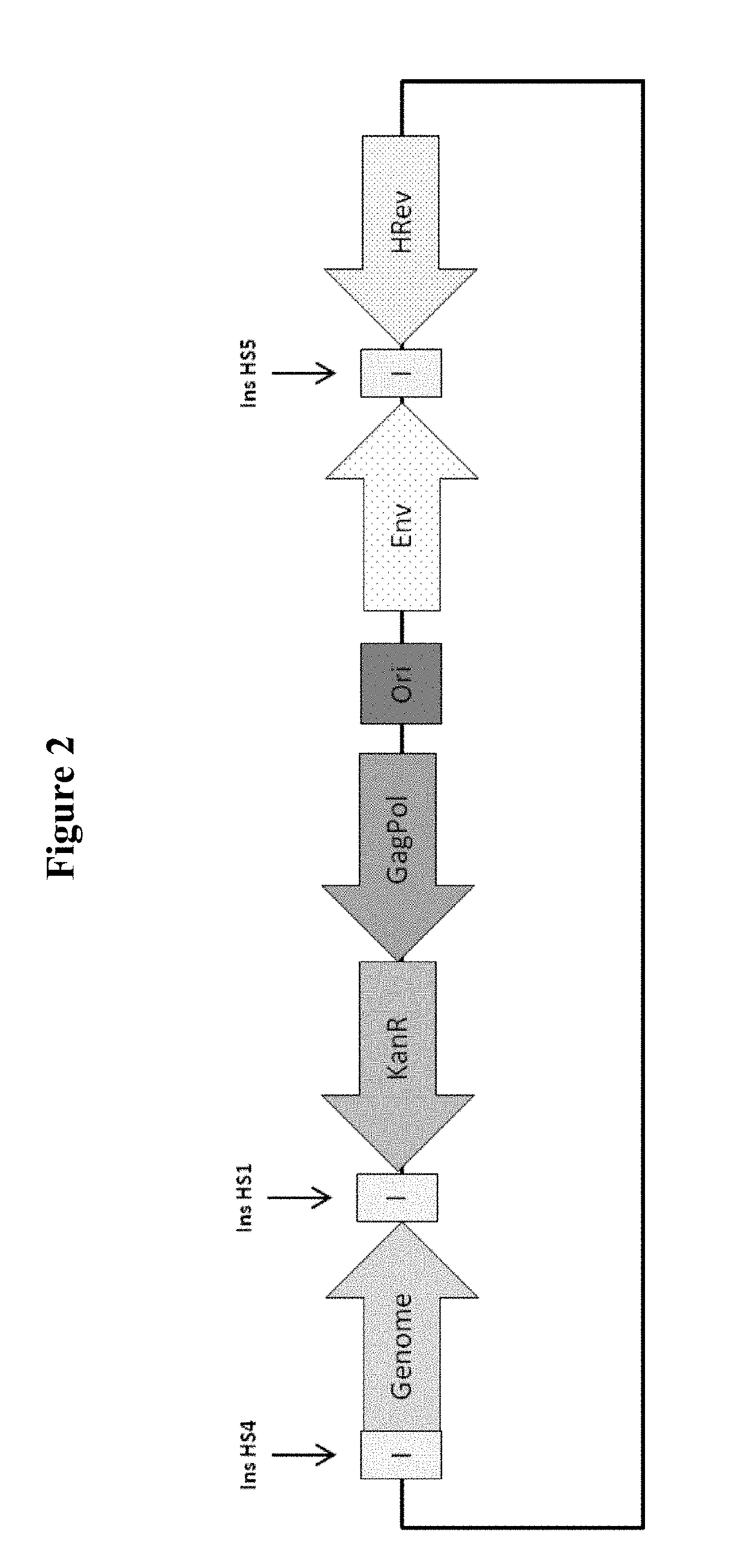
Retroviral Vector
a technology of vectors and retrovirals, applied in the field of retroviral vector production, can solve the problems of genetic instability and poor expression, the size of these constructs could negate the efficiency of cellular entry in the transient and stable vector production system, and increase the chance of the formation of replication-competent viral particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of a Full Transient Modular Plasmid
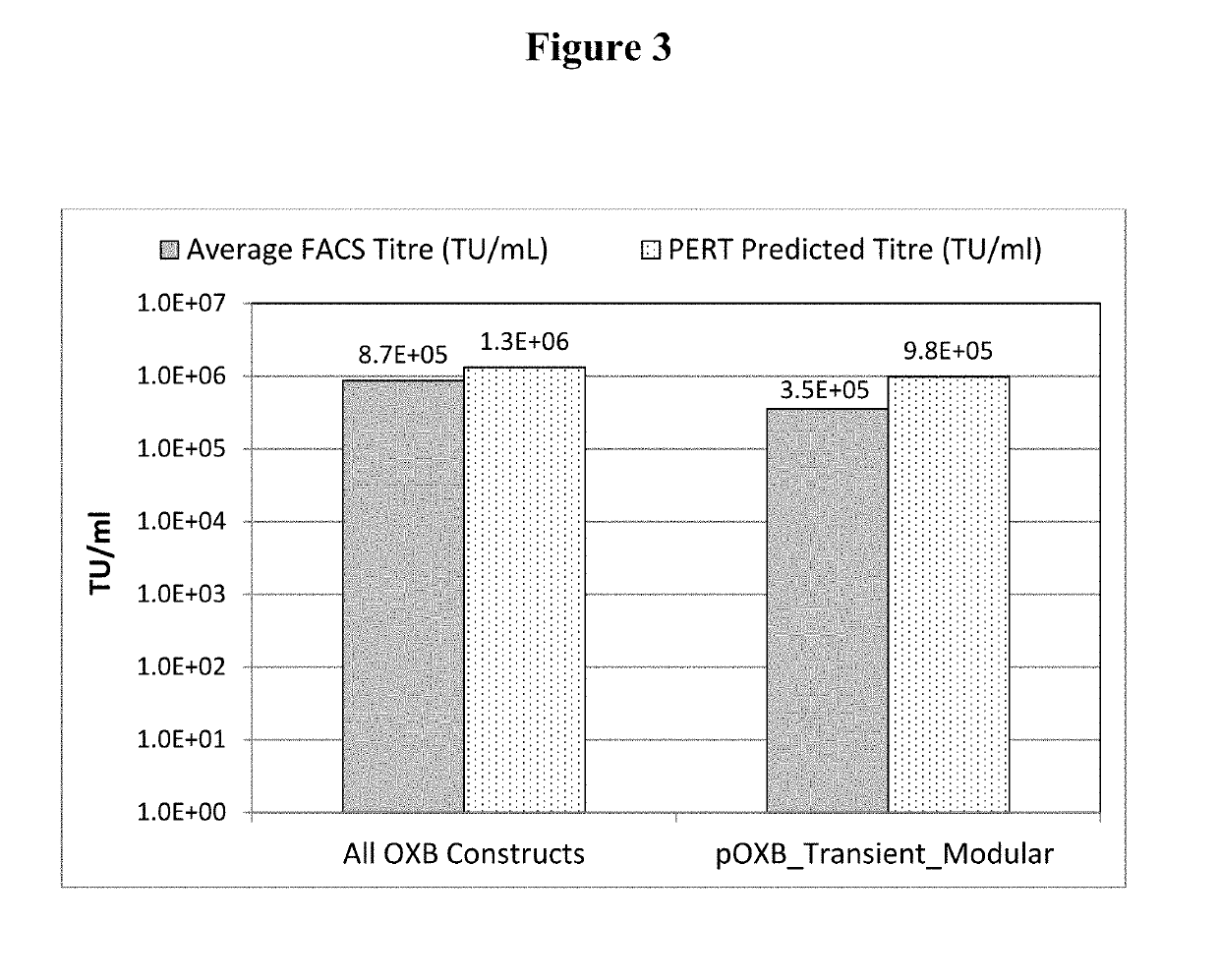
[0347]HIV-1-GFP vector was generated in HEK293T.TetR14 cells (constitutively express the TetR protein) using the full modular DNA constructs (pOXB_Transient_Modular) that is shown in FIG. 2, or by the standard transient co-transfection process using an HIV-1 GFP genome plasmid and three inducible packaging component plasmids (FIG. 1). The standard transient co-transfection process generated vector with an average GFP FACS titre of 8.7E+05 TU / ml. When the full transient modular plasmid was used, vector was generated with an average titre of 3.5E+05 TU / ml, which is only 2.5-fold lower than the standard transient co-transfection process (FIG. 3). These results suggest that the vector titres produced using the multicomponent transient transfection system or the transient modular plasmid are surprisingly similar.
[0348]PERT analysis of vector generated from harvest 2 was normalised to the known reference standard titre thereby generating a PERT predict...
example 2
n of Lentiviral Vector Production Using Modular Constructs in a Transient Transfection Process
[0350]Modular constructs shown in FIG. 5 were evaluated by the transient co-transfection system using the modular construct that was under assessment, plus remaining single component plasmids to complete the vector system. All modular constructs tested in this experiment generated vector with GFP FACS titres above 4E+04 TU / ml, confirming that all modular combinations that had been evaluated were capable of producing lentiviral vector using a transient transfection process. The process of generating GFP vector by transient co-transfection of HEK293T cells with a combination of modular constructs and single component plasmids was not optimised and was based on the optimal transient co-transfection process when four individual plasmids are utilised. Therefore, it is likely that with further optimisation the lentiviral vector yields could be improved. Parameters which may be optimised include: ...
example 3
entiviral Vector Constructs Evaluated Using the Stable Cell Line Process to Generate Pools of Packaging Cell Lines
[0351]Stable pools of packaging cell lines were generated by stable transfection using different combinations of single component plasmids or modular lentiviral vector constructs followed by a period of antibiotic selection. Antibiotic selection ensured selection of only those cells where the lentiviral vector packaging components had been integrated into the host cells genomes. Following a period of cell culture, which ensured all un-integrated plasmid DNAs had been diluted out from the cell cultures, resulting pools of packaging cells were tested for their ability to generate lentiviral vectors containing GFP following transient transfection of HIV-1 GFP genome (and Rev for PAC006) and doxycycline induction. FIG. 6 details the modular construct and single plasmid combinations that were used in the generation of each of the pools of packaging cell lines. In addition, FI...
PUM
| Property | Measurement | Unit |
|---|---|---|
| v/v | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| nucleic acid sequences | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com