Corticosteriod containing foam compositions
a foam composition and corticosteroid technology, applied in the field of foamable compositions including corticosteroid, can solve the problems of corticosteroid degradation over the shelf life of the composition, the conventional method of manufacturing corticosteroid foam, degradation and inactivation, etc., and achieve the effects of enhancing stability, stable ph, and adding manufacturing complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Foamable Halobetasol Propionate Composition
[0067]A composition was prepared in accord with the present invention utilizing the formulation of Table VI above. Listed in Table VI is a specific composition based upon the ranges set forth hereinabove in Table I.
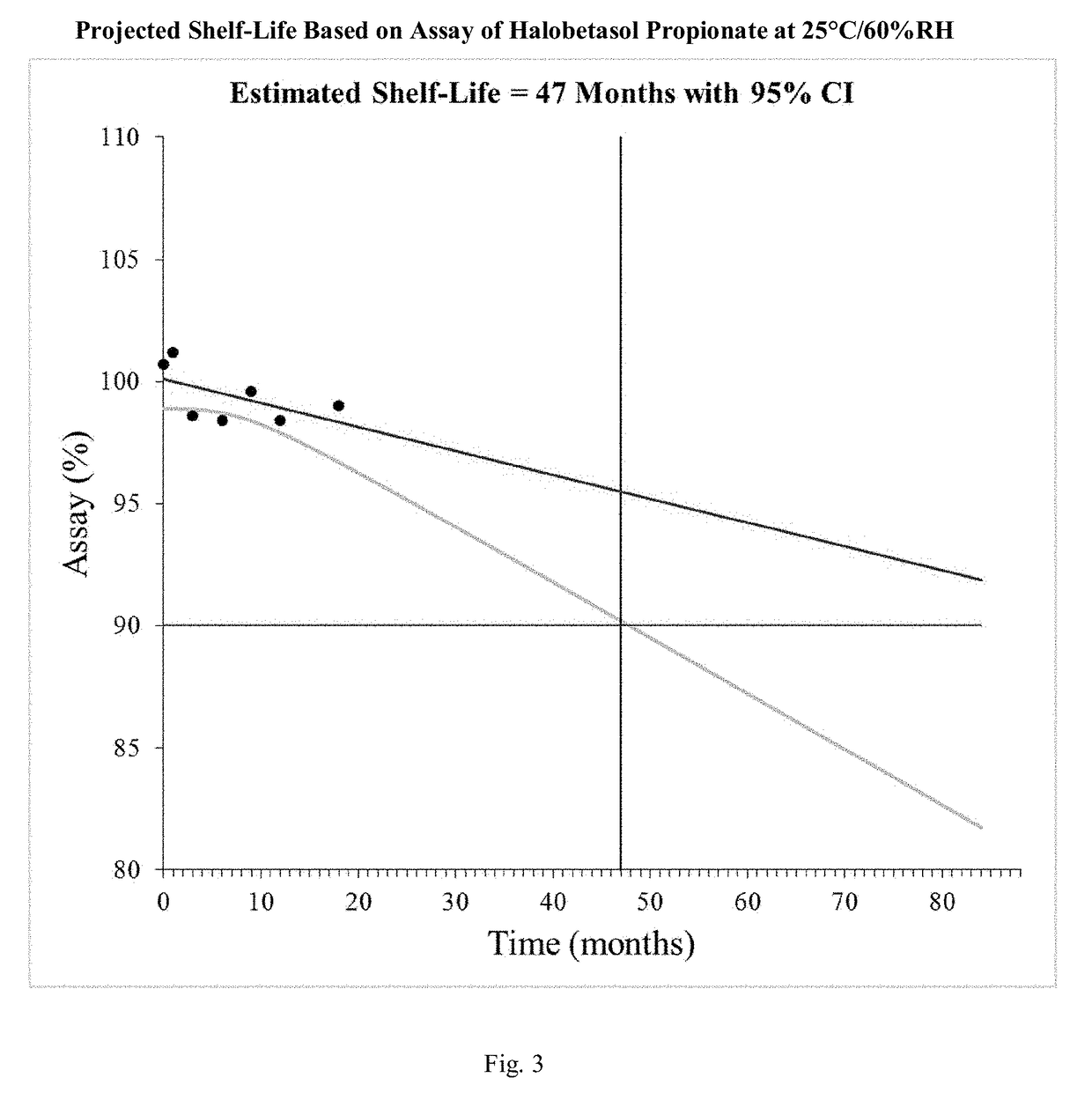
[0068]In this procedure as described in detail herein, a carrier solution is prepared by mixing and heating ethyl alcohol to between about 65°−70° C., adding benzoic acid, propylene glycol, Polyoxyl 20 cetostearyl ether, cetostearyl alcohol, and emulsifying wax and mixing to uniformity while maintaining a temperature of between about 65°−70° C. Water is added in multiple aliquots to maintain a temperature greater than 55° C. and the completed carrier solution is heated to 65°-70° C. The carrier solution is prepared in a vessel with a pressure and / or vacuum rated lid that seals onto the vessel in order to minimize evaporative losses during compounding. Then the carrier is cooled to about 45°−50° C. and the halobetasol propionate i...
example 2
Skin Hydration and Transepidermal Water Loss (TEWL)
[0069]A series of studies was carried out to evaluate the properties and advantages of the composition of the present invention. These studies were carried out utilizing a preparation having a formulation in accord with Table VI as prepared by the procedure set forth above. In a first study, skin hydration was determined by use of an IBS Skicon-200 Conductance Meter equipped with a Measurement Technologies probe [unit 2283, probe A] to further enhance its ability to measure changes in skin surface hydration.
[0070]The data confirm that the composition of the present invention did not increase skin hydration when applied to shaved skin and was not considered to be occlusive. In fact, the composition decreased skin hydration (i.e., dehydrated) when applied to shaved skin.
[0071]A further study was carried out measuring transepidermal water loss (TEWL) of skin treated with the composition of the present invention. Computerized evaporimet...
example 3
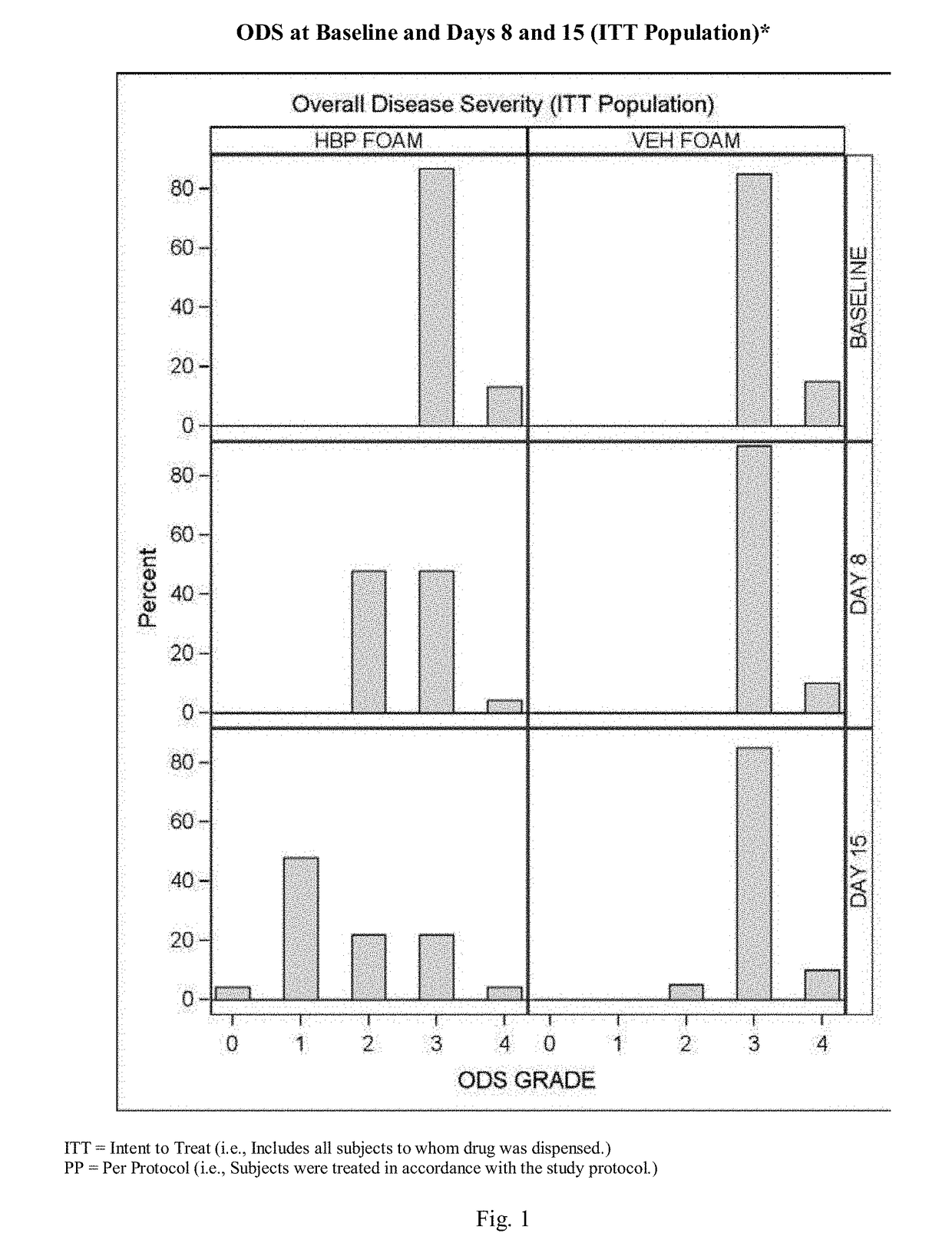
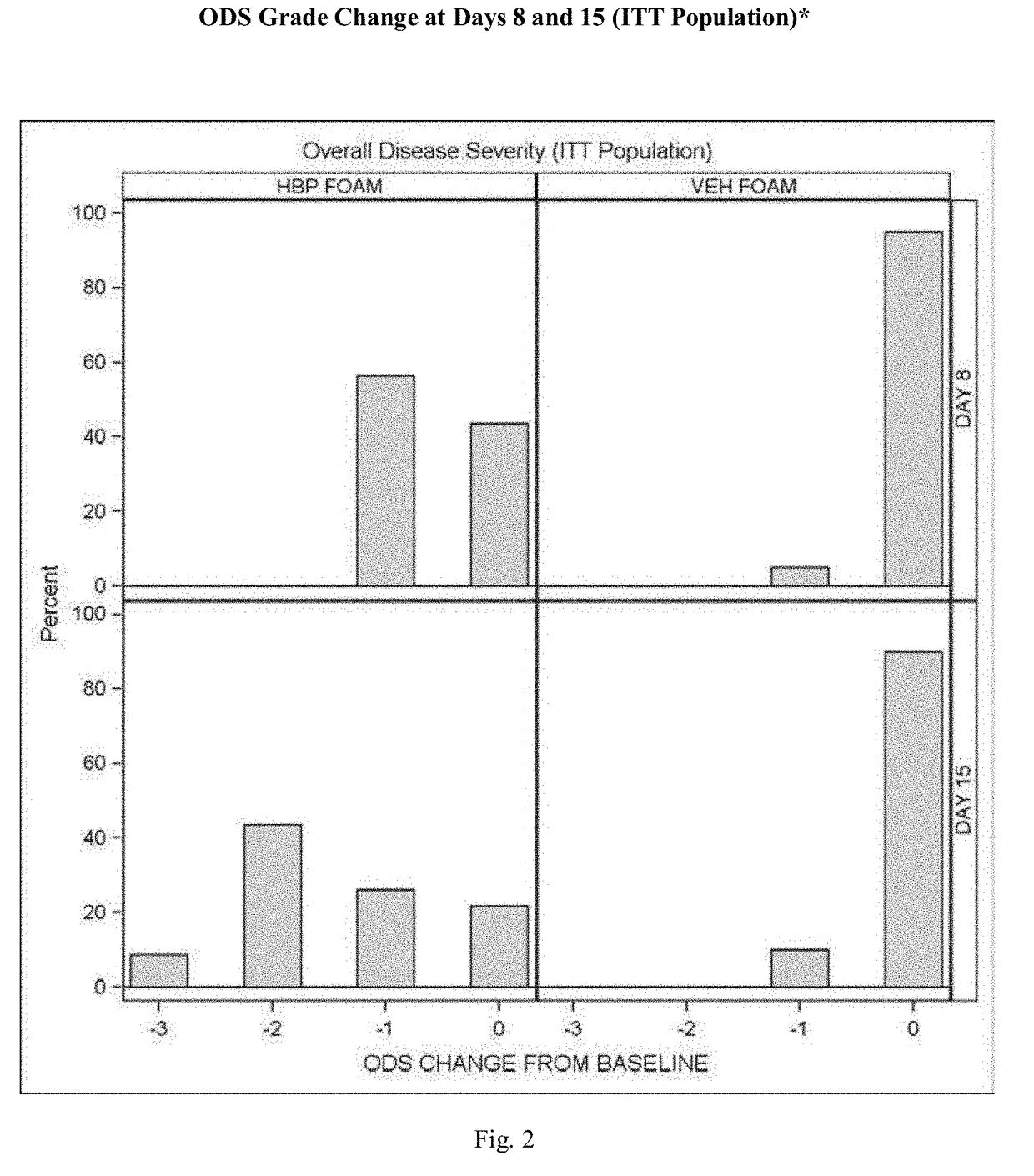
[0075]A further experimental study evaluated the clinical efficacy of the composition of the present invention having the formulation of Table VI, described above, in the treatment of subjects with plaque psoriasis.
[0076]Results
[0077]52% of psoriasis subjects treated with the composition of the present invention having the formulation described above, and 0.0% of subjects treated with an identical composition void of halobetasol (Control Vehicle), achieved “treatment success”.
Definitions
[0078]Overall Disease Severity (ODS): At every visit, the overall severity of a subject's psoriasis in the Treatment Area, taking into consideration the three individual clinical signs of psoriasis (scaling, erythema, and plaque elevation) was assessed using a five-point scale ranging from 0=clear to 4=severe / very severe. To be enrolled in the study the subjects had to have at least a moderate ODS score (≥3).
Clear (0)ScalingNo evidence of scaling.ErythemaNo erythema (hyperpigmentatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com