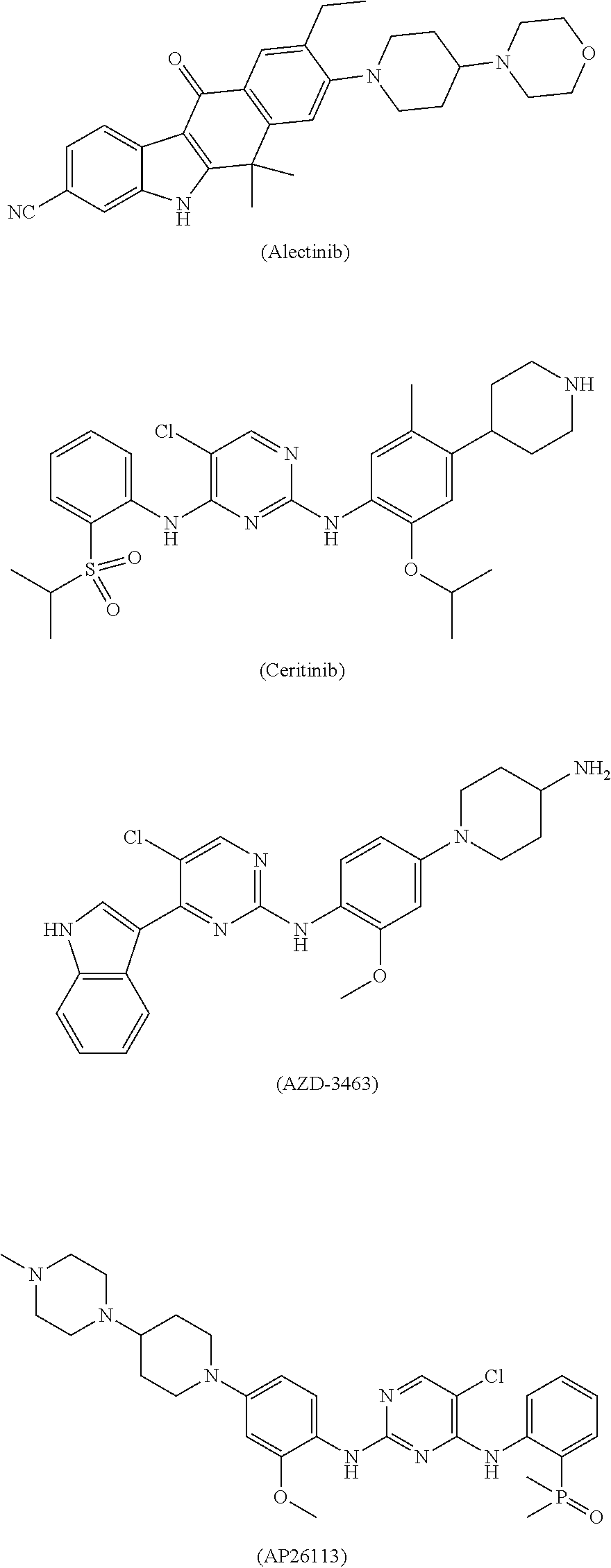
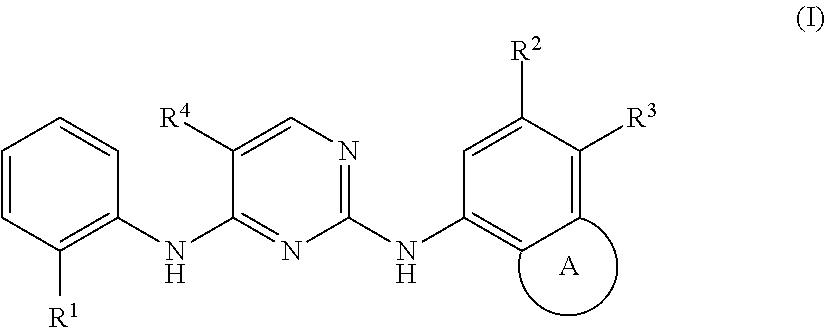
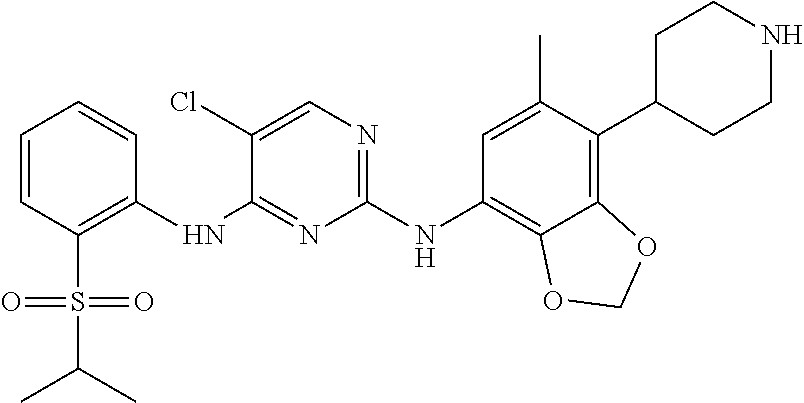
Polycyclic inhibitor of anaplastic lymphoma kinase
a polycyclic inhibitor and lymphoma kinase technology, applied in the field of polycyclic inhibitors of anaplastic lymphoma kinase, can solve the problems of malignant cell transformation, high expression and over-activation of fusion proteins having alk kinase activity,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example 1
Assay on In Vitro ALK Kinase-Inhibiting Activity of the Compounds Provided in Examples of the Invention
[0171]Test compounds: Compounds 1, 2, 3 and 4provided in Examples of the invention, the chemical names and preparation methods of which can be found in the preparation examples.
[0172]Control agent: Ceritinib, lab-made (prepared by reference to the method in Publication WO2008 / 073687A2).
[0173]Experimental Method
[0174]Preparation of ALK Kinase Buffer: A suitable amount of a stock solution of MgCl2 at a concentration of 1000 mM, a suitable amount of a stock solution of SEB at a concentration of 2500 nM, a suitable amount of a stock solution of DTT at a concentration of 100 mM, and a suitable amount of 5×enzyme buffer were added to ultrapure water to reach a final concentration of: 5 mM, 25 nM, 1 mM, and 1×enzyme buffer, respectively. The resultant mixture was mixed homogeneously, for later usage.
[0175]Preparation of 2.5×Test Compound Colutions:
[0176]Preparation of a stock solution of ...
experimental example 2
Assay on In Vitro ALK Kinase-Inhibiting Activity of the Compounds Provided in Examples of the Invention
[0190]Test compounds: Compounds 5, 6 and 8 provided in Examples of the invention, the chemical names and preparation methods of which can be found in the preparation examples.
[0191]Control agent: Ceritinib, lab-made (prepared by reference to the method in Publication WO2008 / 073687A2).
[0192]Experimental method: Measurement of ALK kinase inhibitory activity by Caliper Mobility Shift assay.
[0193]1. Preparation of 1-Fold Kinase Buffer
[0194]To pH7.5HEPES, Brij-35 at a concentration of 30%, a stock solution of MgCl2 at a concentration of 1M, and a stock solution of DTT at a concentration of 1M, ultrapure water was added and mixed homogeneously until HEPES was at a final concentration of 50 mM, Brij-35 was at a final concentration of 0.0015%, MgCl2 was at a final concentration of 10 mM, and DTT was at a final concentration of 2 mM.
[0195]2. Preparation of Stop Solution
[0196]To a stock solu...
experimental example 4
Assay on In Vitro Cell Activity of the Compounds Provided in Examples of the Invention
[0231]Test compounds: Compounds 1-6 and 8 provided in Examples of the invention, the chemical names and preparation methods of which can be found in the preparation examples.
[0232]Control agent: Ceritinib, lab-made (prepared by reference to the method in Publication WO2008 / 073687A2), the formula of which is shown in the Background.
[0233]The meanings of abbreviations in the following experiments are described as follows.
[0234]rpm: revolutions per min;
[0235]DMSO: dimethyl sulfoxide;
[0236]MTS: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazoliumbromide;
[0237]RPMI1640: 1640 medium (RPMI: Roswell Park Memorial Institute);
[0238]“×” in 500×, 1000×, 10×: fold.
[0239]Experimental Method
[0240](I)NCI-H3122, Karpas-299cell:
[0241](1) Cell Preparation:
[0242]The cells were cultured to a fusion degree of 80% in RPMI-1640 medium containing 10% fetal bovine serum, 100 U / ml penicillin and 100 mg / ml streptomycin, i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com