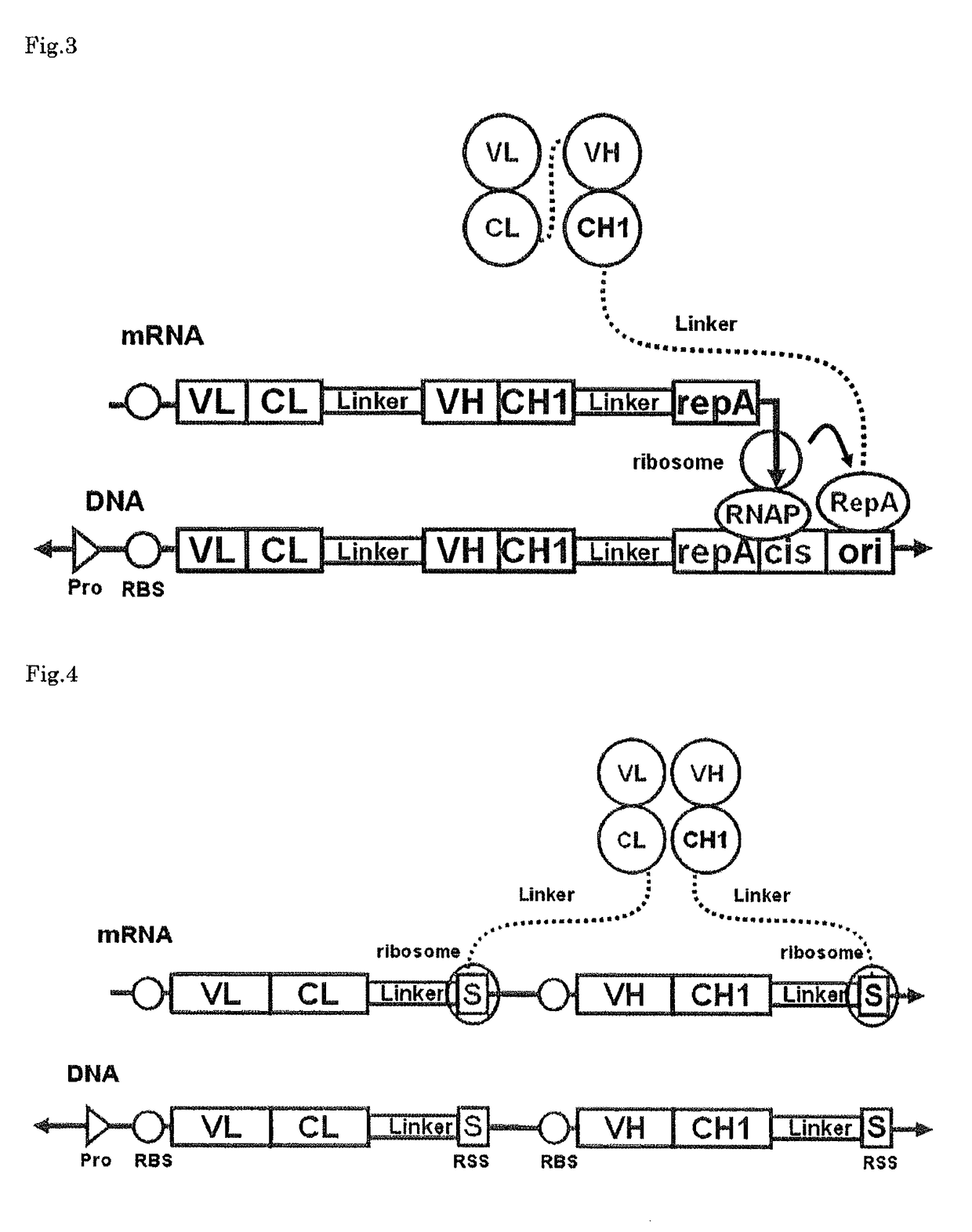
Polynucleotide construct capable of displaying fab in a cell-free translation system, and method for manufacturing and screening fab using same
a technology of cell-free translation and polynucleotide constructs, which is applied in the direction of immunoglobulins, instruments, peptides, etc., can solve the problems of difficult efficient screening of fabs composed of two peptide chains and large molecular weight, and achieves easy construction, simple operation, and short screening period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[Example 1] Preparation of Model Fab
[0270]As the framework of the Fab to be used in the cell-free Fab display system, the combination of VL k subgroup I and VH subgroup III, which is one of the major antibody subclasses in human, excellent in expression efficiency in E. coli (Kanappik et al., J Mol Biol. (2000) vol. 296, p. 57-86) and reported to be effective in a cell-free scFv display system (Shibui et al., Appl Microbiol Biotechnol. (2009) vol. 84, p. 725-732), was selected. As a model Fab having the above-described framework to be used for confirmation of the performance of the cell-free Fab display system, the human EGFR-2 (Her-2)-reactive Fab reported by Carter et al. (Carter et al., Proc Natl Acad Sci USA. (1992) vol. 89, p. 4285-4289) was selected to provide Fab-HH.
[0271]As another model Fab, Fab-TT was provided by substituting the CDR 1-3 regions in the L chain and the H chain of Fab-HH by the sequence of the INFαR-reactive scFv discovered by the cell-free scFv display syst...
example 2
[Example 2] Evaluation of Activities, Specificities and L Chain-H Chain Interdependencies of Model Fabs
[0275]Each of Her-2 and INFαR (R&D systems) as an antigen protein for the model Fab was diluted with PBS to 1 μg / ml. The diluted antigen was placed on an ELISA plate, and immobilized on the plate by incubation at 4° C. overnight. After washing the well, the model Fab was added thereto to bind the model Fab to the antigen. After washing the well, a 1000-fold diluted HRP-labeled anti-FLAG antibody (Sigma) as a detection antibody was added to the well, to bind the detection antibody to the FLAG tag attached to the model Fab. After washing the well, a colorimetric substrate for HRP was added thereto. When an appropriate degree of coloring was achieved, a stop solution was added to the well, and the absorbance was measured at OD 405 nm. FIG. 11 shows the result of titration of the 4 types of model Fabs over a wide range of concentration using Her-2 as the antigen protein. It can be seen...
example 3
[Example 3] Construction of Vector for Bicistronic Fab-PRD
[0276]A sequence having a very similar amino acid sequence to that of the above-described Fab-HH and reported by Fellouse et al. (Fellouse et al., J. Mol. Biol. (2007) vol. 373, p. 924-940) to show even higher expression efficiency in E. coli was provided as Fab-SS. For the display of this sequence in a cell-free system, a DNA fragment for bicistronic Fab-PRD was artificially synthesized using codons optimized for E. coli. The synthesized fragment was incorporated into a general-purpose phagemid vector pBluescript SK(+) to construct pGAv2-SS. Subsequently, the CDR 1-3 regions in the L chain and the CDR 1-3 regions in the H chain of pGAv2-SS were replaced by the corresponding regions in pTrc Fab-HH, to construct pGAv2-HH. Similarly, using pGAv2-SS and pTrc Fab-TT, pGAv2-TT was constructed. Subsequently, by introducing a XhoI recognition site into the most downstream part of the GS linker downstream of the L chain of pGAv2-HH w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com