Method and Apparatus for Manufacturing, Filling and Packaging Medical Devices and Medical Containers
a technology for medical devices and containers, applied in the direction of packaging goods, transportation and packaging, liquid materials, etc., can solve the problems of increasing the time and difficulty of filling the syringe, increasing the possibility of drug solution contamination, and glass particles from the ampoules contaminating the drug solution, etc., to achieve low bio-burden, maintain cleanliness, and low bio-burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
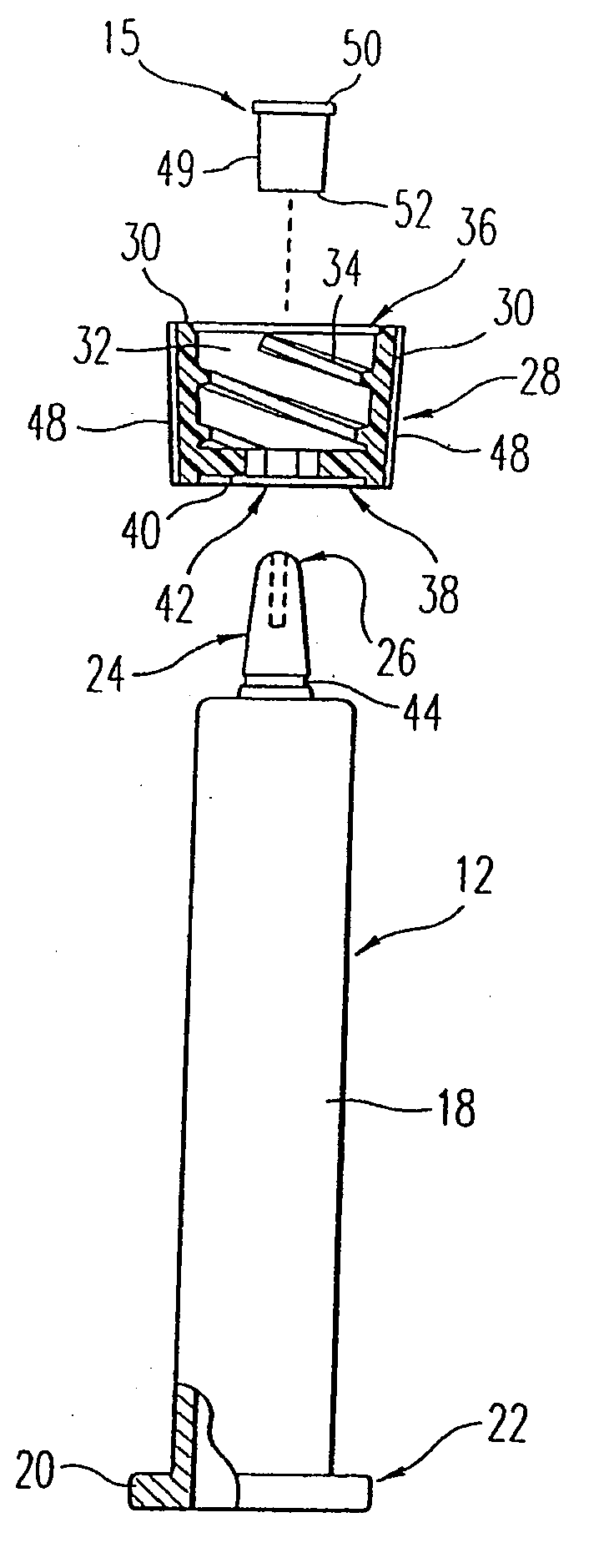
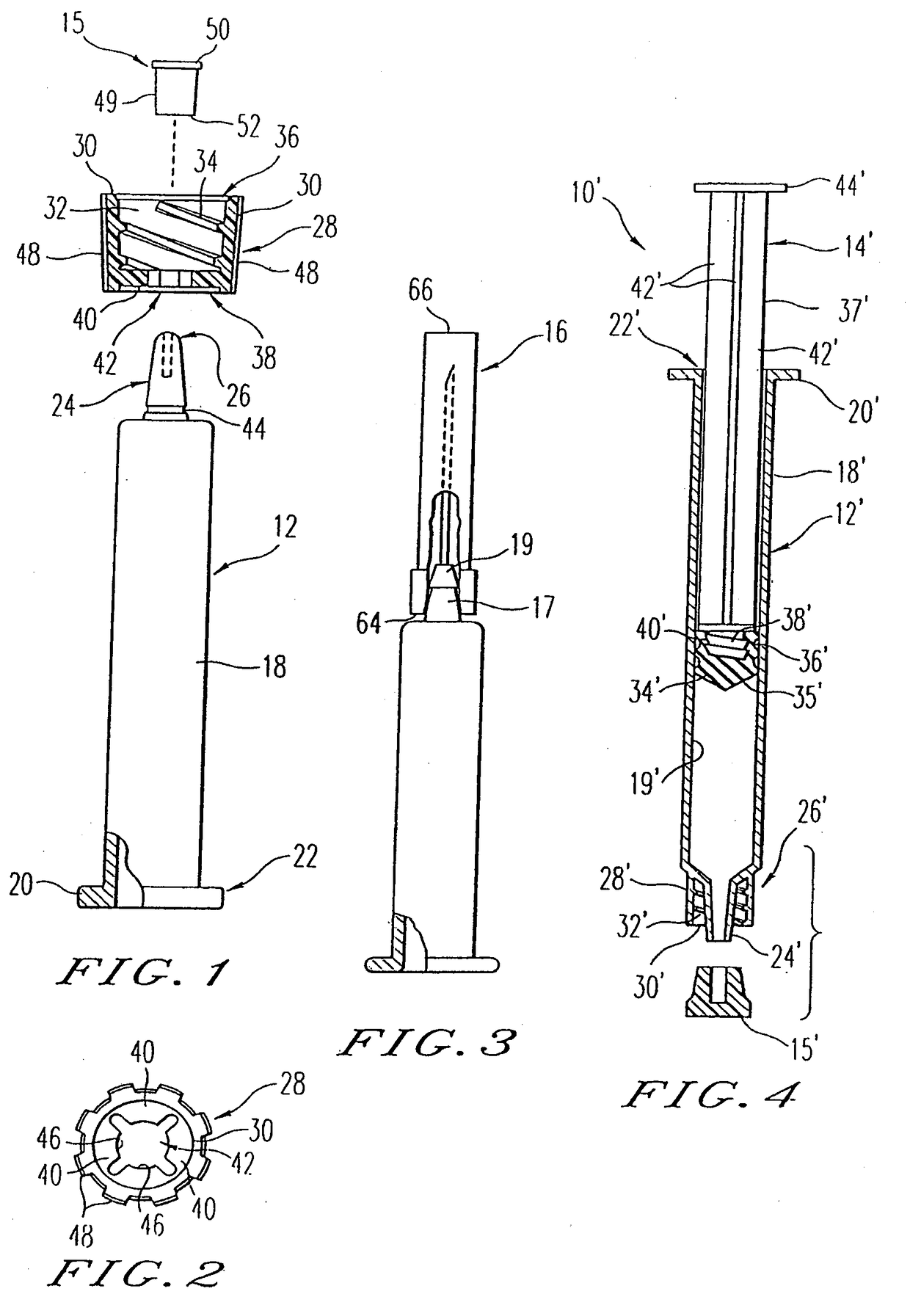
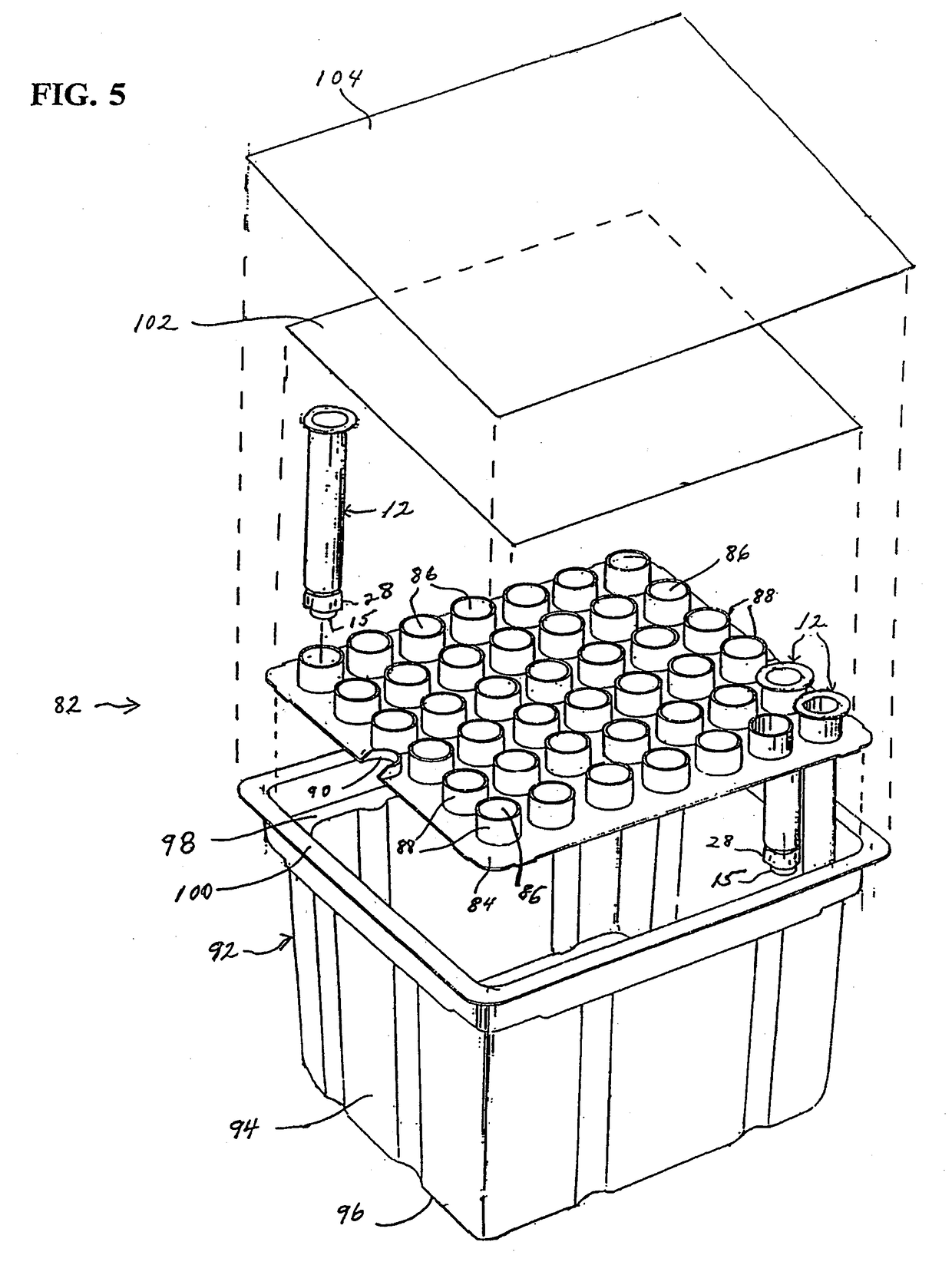
[0034]The present invention is directed to a method and apparatus for manufacturing and thereafter assembling and packaging medical containers, drug delivery devices and drug containers, such as vials, syringe barrels and prefilled syringes, in a clean, environmentally controlled area. As used herein, medical containers for containing and / or dispensing substances include vials and injection devices such as syringes. In addition, as used herein, a substance includes, for example, water, saline solutions, flush solutions and contrasting agents, pharmaceutical agents and vaccines in either a dry state or liquid state. The medical containers can be syringe barrels formed from a base material such as glass or plastic. The syringe barrels are used to assemble a syringe 10 as shown in FIG. 1. Although embodiments of the invention are disclosed as a hypodermic syringe assembly, it is within the purview of the present invention to include various other drug containers, such as plastic or gla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com