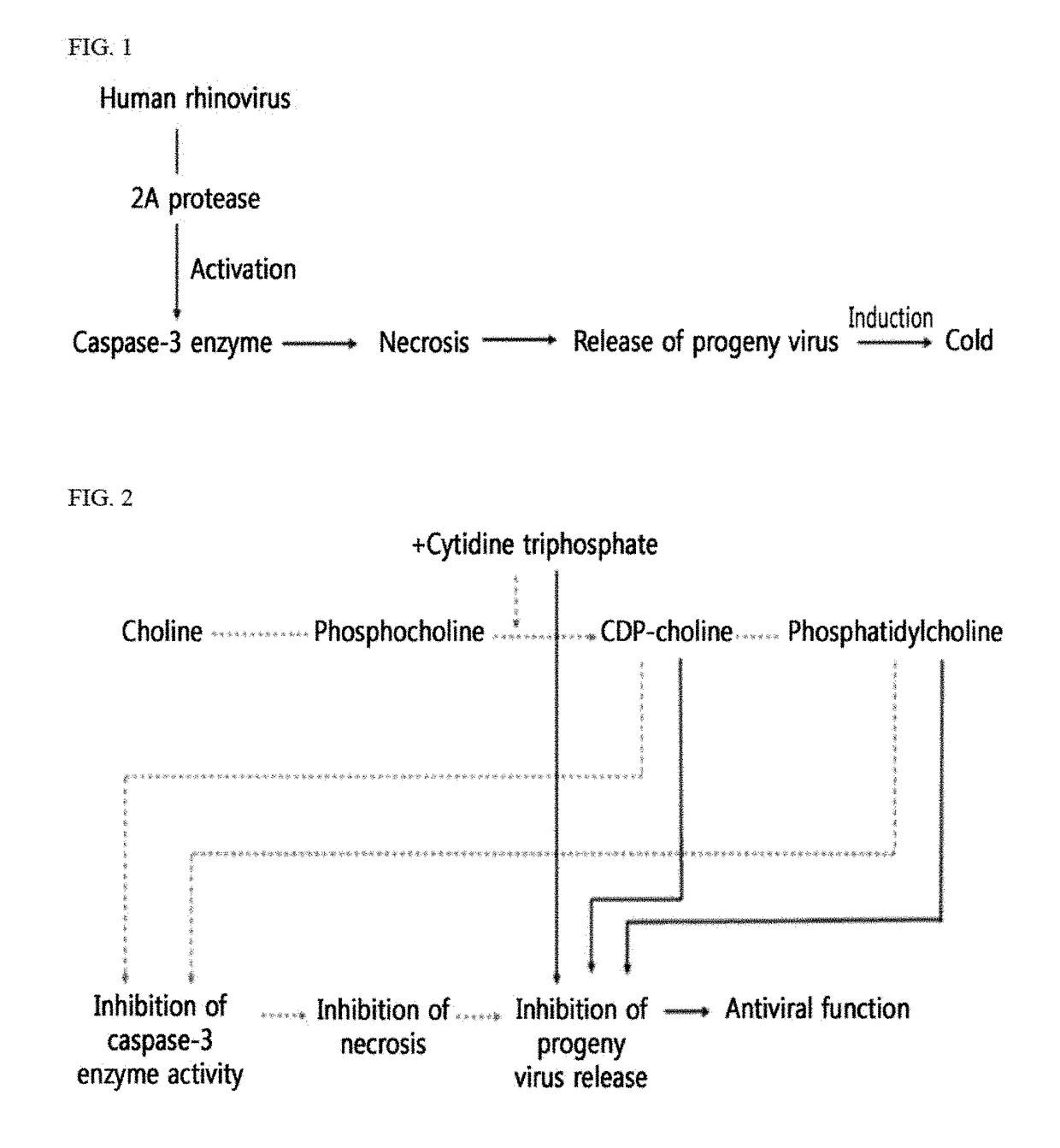
Antiviral composition containing material involved in phosphatidylcholine snythesis pathway
a technology of phosphatidylcholine and antiviral composition, which is applied in the field of composition, can solve the problems of low effect of pirodavir, inability to develop therapeutic agents against i>rhinovirus /i>infection, and low pharmacokinetic value of pirodavir, so as to prevent or improve viral diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antiviral Activity Test Against Rhinovirus
[0088]HeLa cells (2×104 cells) were placed in each well of 96-well plates and cultured for 24 hours. After 24 hours, the culture supernatant of each well was removed, and then rhinovirus type 2, type 3, and type 5 solutions titrated with TCID50 were placed in each well. The virus used in the present invention was purchased from the American Type Culture Collection (ATCC). Each virus activity thereof was cultured and stored at −70° C. for use.
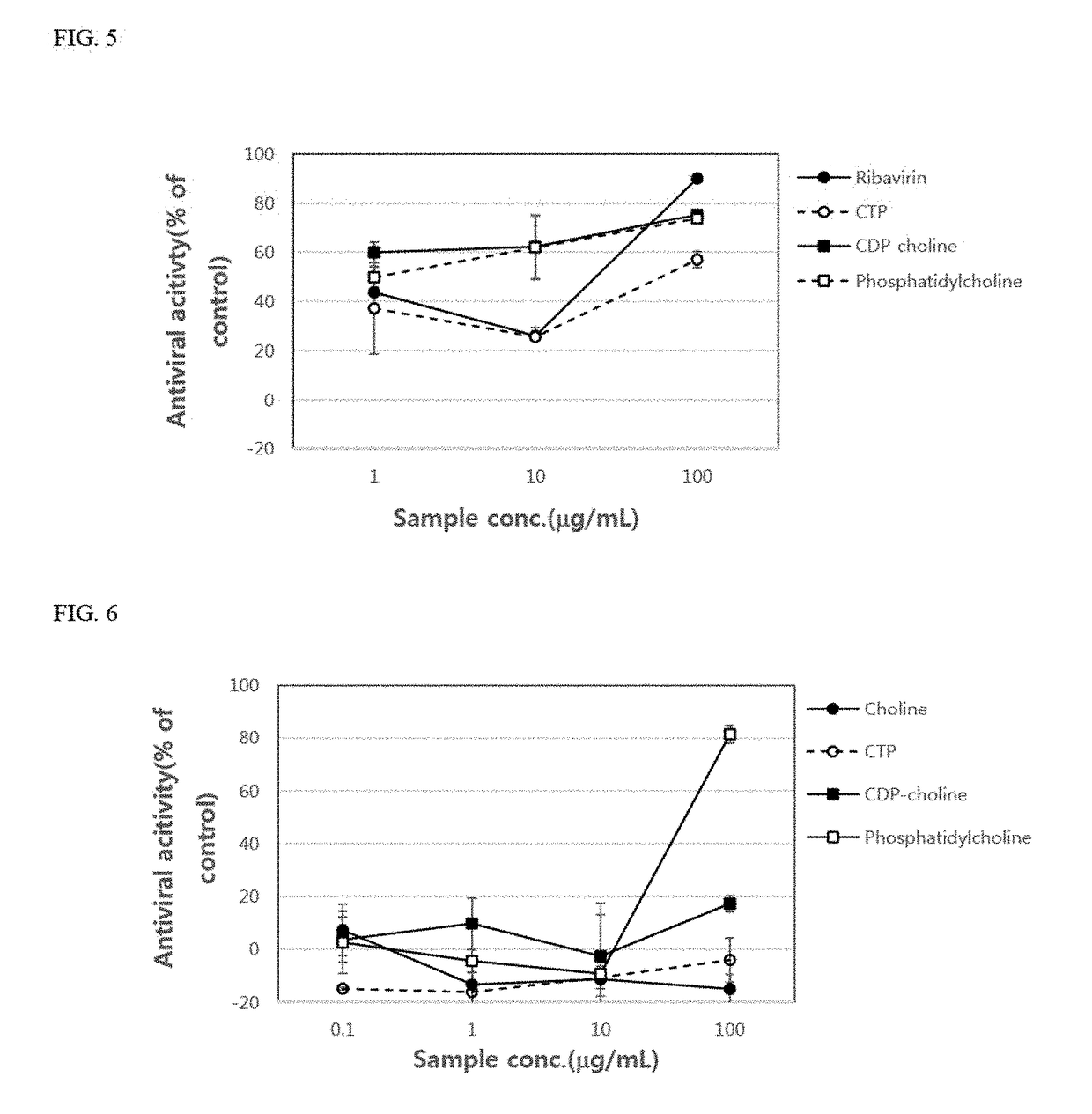
[0089]Ribavirin, which is a control group, cytidine triphosphate, CDP-choline, phosphatidylcholine, and adenosine triphosphate, which are experimental groups, were treated in each well in amounts of 0.1 μg / ml, 1 μg / ml, 10 μg / ml, and 100 μg / ml, respectively. Each compound was purchased from Sigma Corp.
[0090]The anti-proliferative ability against a virus was measured according to the methods described in Korean Patent No. 10-0682069. Specifically, after completion of the viral infection test, a 70% aceton...
example 2
Antiviral Activity Test Against Enterovirus
[0100]Vero cells (2×104 cells) were placed in each well of 96-well plates and cultured for 24 hours. After 24 hours, the culture supernatant of each well was removed, and then coxsackievirus type A solution or type B solution, which is a type of enterovirus, titrated with TCID50 was placed in each well. In addition, as shown in FIG. 6, choline, cytidine triphosphate, CDP-choline, and phosphatidylcholine were added thereto at concentrations of 0.1 μg / ml, 1μg / ml, 10 μg / ml, and 100 μg / ml. As a result, it was confirmed that the antiviral activity of cytidine triphosphate, CDP-choline, and phosphatidylcholine increased as the concentration thereof increased. Further, it was also confirmed that the antiviral activity of phosphatidylcholine was significantly remarkable at the concentration of 100 μg / ml compared to that of ribavirin, an antiviral agent used as the control group (Table 2).
TABLE 2Drug concentration (100 μg / mL)CoxsackievirusCoxsackie...
example 3
Inhibitory Activity of Viral RNA Production
[0103]In order to confirm mechanism of the composition of the present invention, which inhibits the necrosis of virus-infected cells, the following experiment was conducted.
[0104]In FIG. 8-A, HeLa cells were cultured in a 6-well plate, followed by infecting rhinovirus type 2 with TCID50. Thereafter, ribavirin, CDP-choline, phosphatidylcholine, ATP, and CTP were added to each well, and then RNA was respectively isolated from the cells in each well after 48 hours. cDNA was prepared using reverse transcriptase from the same amount of the extracted RNA, and beta-actin genes (300 bp) were amplified using a beta-actin-specific primer set. The result thereof is represented in FIG. 8-A, and it was confirmed that beta-actin genes in all wells were amplified in similar amounts.
[0105]In FIG. 8-B, rhinovirus genes (188 bp) were amplified using a rhinovirus-specific primer from cDNA previously prepared. The result thereof is represented in FIG. 8-B. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com