Composition for treating lung cancer, particularly of non-small lung cancers (NSCLC)
a technology for lung cancer and composition, applied in the field of active composition, can solve the problems of uncontrollable difficult to rule out the risk of uncontrolled propagation of introduced genes and viral genes, and poor approach to nsclc, etc., and achieve the effect of stimulating the immune system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0602]The following examples are intended to illustrate the invention further. They are not intended to limit the subject matter of the invention thereto.
1. Preparation of Encoding Plasmids:
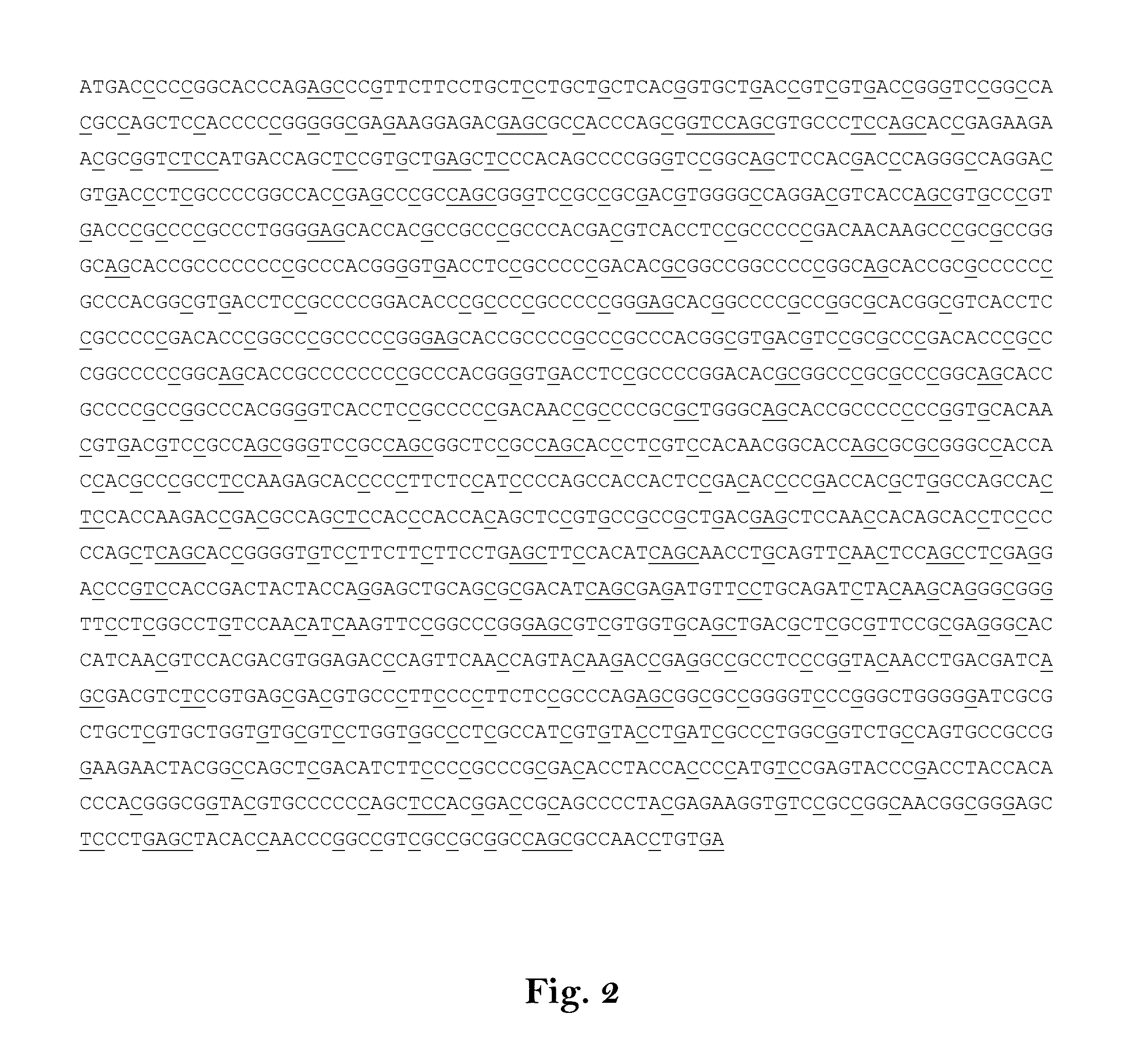
[0603]In the following experiment DNA sequences, corresponding to the respective mRNA sequences end encoding the antigens[0604]hTERT,[0605]WT1,[0606]MAGE-A2,[0607]5T4,[0608]MAGE-A3,[0609]MUC1,[0610]Her-2 / neu,[0611]NY-ESO-1,[0612]CEA,[0613]Survivin,[0614]MAGE-C1, or[0615]MAGE-C2.
[0616]respectively, were prepared and used for in vitro transcription and transfection experiments. Thereby, the DNA sequence corresponding to the native antigen encoding mRNA was increased in GC-content and codon-optimized. Then, the coding sequence was transferred into an RNActive construct (CureVac GmbH, Tübingen, Germany), which has been modified with a poly-A-tag and a poly-C-tag (A70-C30).
2. In Vitro Transcription:
[0617]Based on the recombinant plasmid DNA obtained in Example 1 the RNA sequences were prepared by in v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| mass ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com