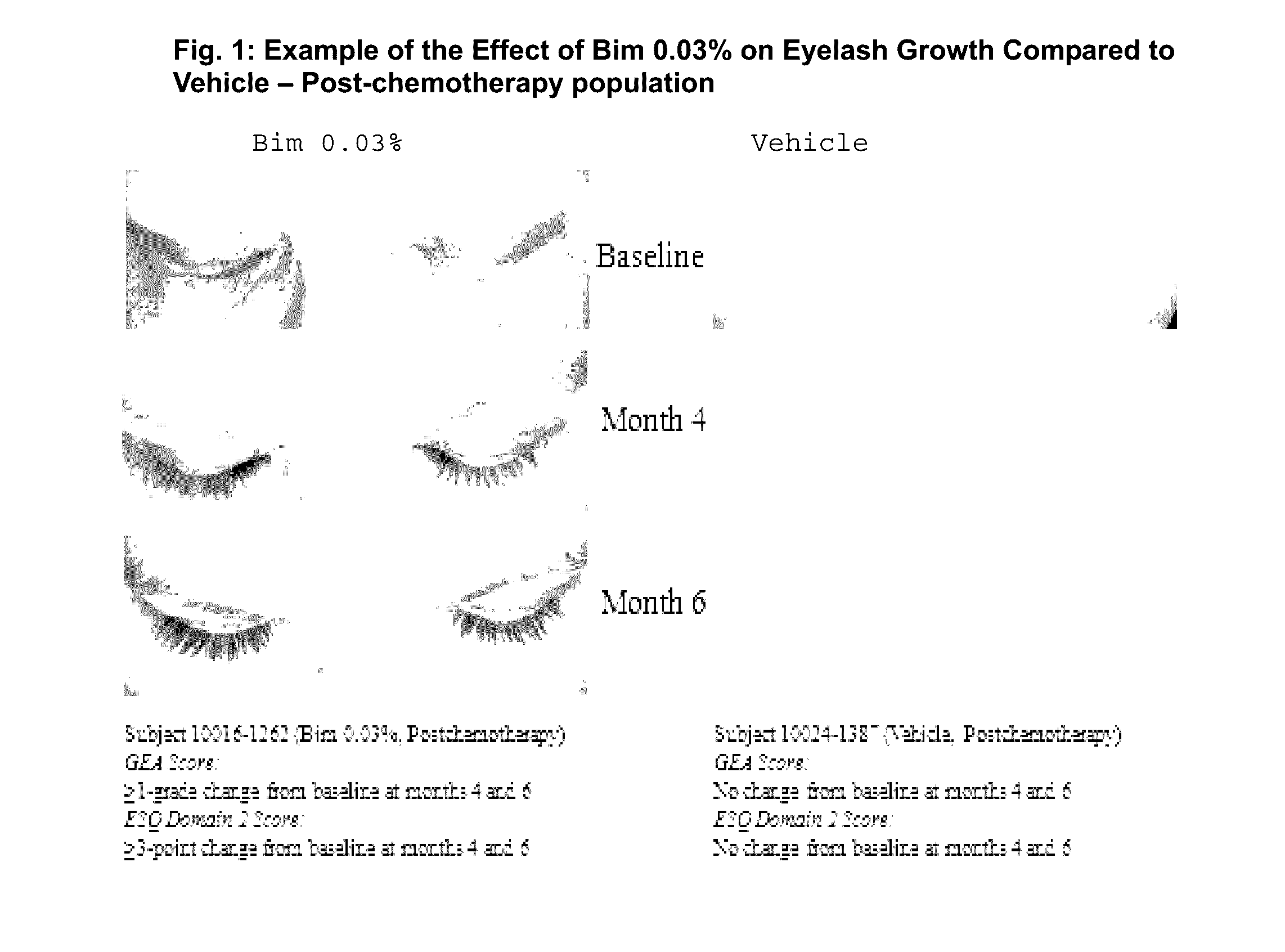
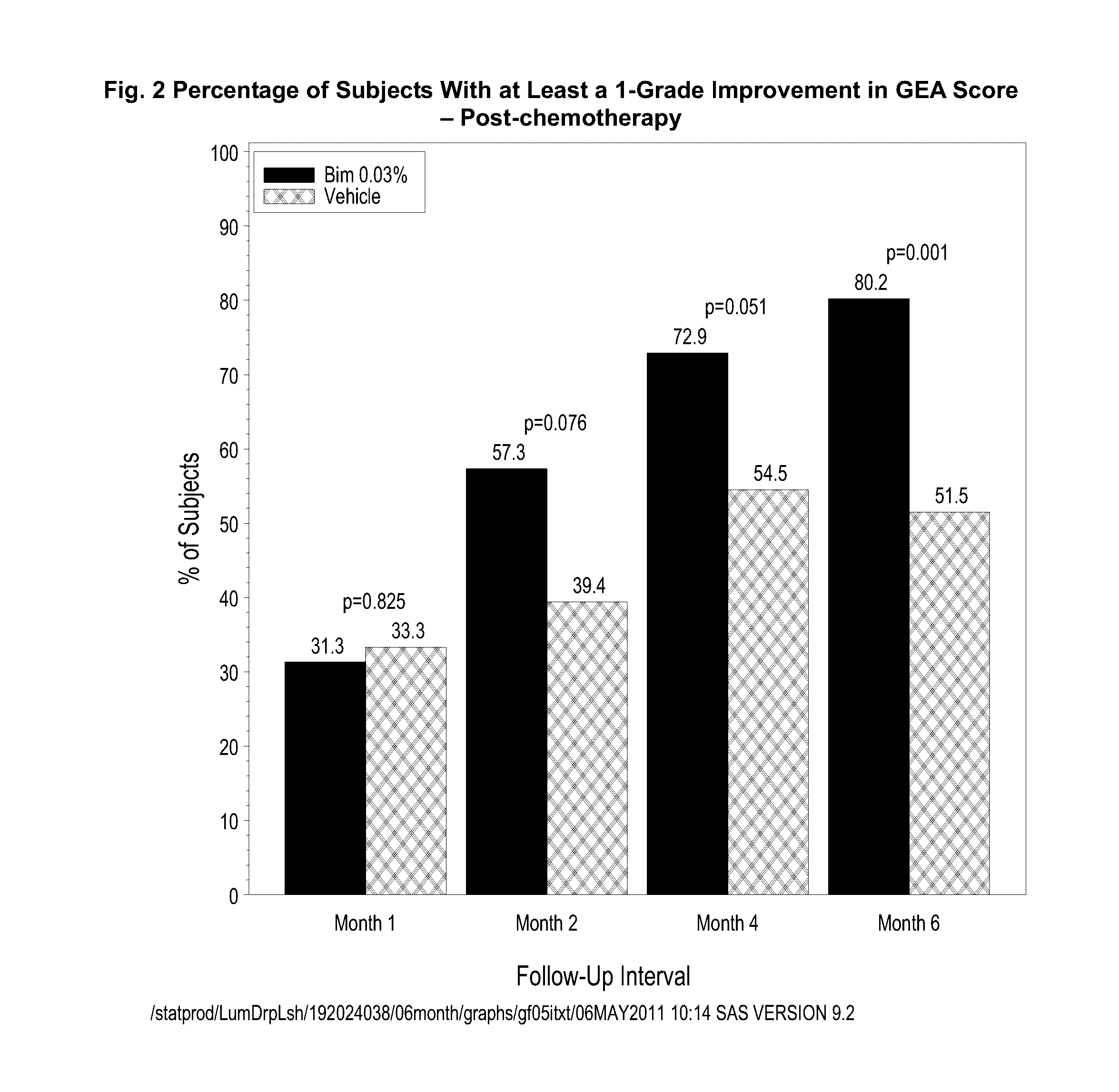
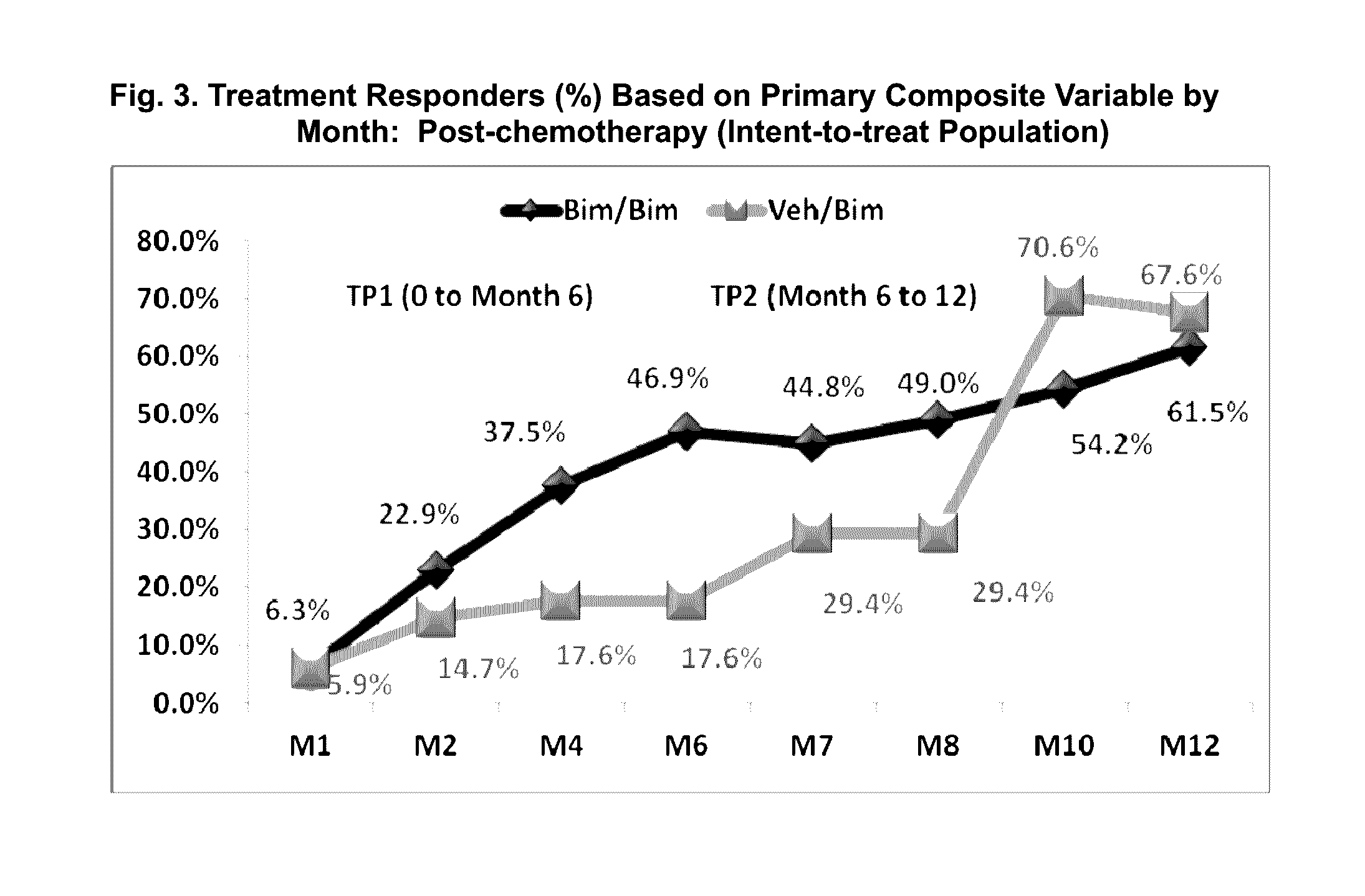
Topical treatment for chemotherapy induced eyelash loss or hypotrichosis using prostamide f2 alpha agonists
a technology of prostamide and alpha agonist, which is applied in the field of postchemotherapeutic hypotrichosis treatment, can solve the problems of chemotherapy-induced hair loss, patchy hair loss, damage to the hair follicle components, etc., and achieve the effect of preventing the loss of eyelashes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0057]
TABLE IList of Components and Quantitative CompositionConcen-Concen-trationtrationIngredients(% w / v)(mg / mL)FunctionActive ingredientBimatoprosta0.030.3ActiveingredientOther ingredientsBenzalkonium chlorideb0.0050.05PreservativeSodium phosphate0.2682.68BufferingdibasicheptahydrateagentCitric acid monohydrate0.0140.14BufferingagentSodium chloride0.838.3TonicityagentHydrochloric acidc and / or sodiumAdjust to pH 7.2-7.4pH adjusterhydroxidecPurified waterq.s. adq.s. adVehicle100%1 mL
Clinical Data:
[0058]A clinical study was conducted that demonstrated the clinical benefits of bimatoprost 0.03% solution in treating eyelash loss resulting from chemotherapy treatment.
Study Design and Structure:
[0059]This was a 1-year, multicenter, double-masked, randomized, parallel-group study to evaluate the safety and efficacy of bimatoprost solution 0.03% in increasing overall eyelash prominence following dermal application to the upper eyelid margins in normal adults and postchemotherapy adults exh...
example 2
[0086]This is a long-term safety and efficacy study of bimatoprost ophthalmic solution 0.03% (LATISSE®) bimtoprost carried out in idiopathic and post-chemotherapy hypotrichosis populations. In this study, eyelash loss from chemotherapy was studied.
Study Design:
[0087]A one-year, multicenter, randomized, double-masked, vehicle-controlled study. Adult post-chemotherapy and idiopathic eyelash hypotrichosis subjects were enrolled based on their score of 1 or 2 on a four point ordinal Global Eyelash Assessment (GEA) scale, and in addition having a low score on a PRO measure associated with ‘psychological impact’ of the condition, a domain-2 of the Eyelash Satisfaction Questionnaire (ESQ). The study involved two treatment periods of six months each. In the first treatment period, subjects for both populations were randomized 3:1 for QD bimatoprost: vehicle treatment. In the second 6-month treatment period, all subjects were moved to bimatoprost treatment, except for a group of bimatoprost ...
example iii
Objective:
[0100]To evaluate long-term safety and efficacy of bimatoprost among subjects with idiopathic or chemotherapy-induced hypotrichosis.
Methods:
[0101]This multicenter, double-masked, randomized, parallel-group study included two 6-month treatment periods. Subjects with idiopathic hypotrichosis were randomized to 3 treatment groups: 1) treatment period 1 (TP1) and TP2: bimatoprost; 2) TP1: bimatoprost; TP2: vehicle; and 3) TP1: vehicle; TP2: bimatoprost. Subjects with chemotherapy-induced hypotrichosis were randomized to 2 treatment groups: 1) TP1: bimatoprost or vehicle; and 2) TP2: bimatoprost. The primary endpoint was a composite of at least a 1-grade improvement in investigator-assessed Global Eyelash Assessment (GEA), and at least a 3-point improvement in subject-reported Eyelash Satisfaction Questionnaire (ESQ) Domain 2 (self-perceived confidence, attractiveness, and professionalism) at month 4. Secondary measures included digitally assessed eyelash characteristics (i.e.,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com