Composition containing herbal medicine extracts of pinellia and scutellaria for reducing side effects due to anticancer drugs
a technology of anticancer drugs and herbal medicine, applied in the field of composition, can solve the problems of further increasing indigestion or compaction, reducing gastrin production, and gastrointestinal toxicities, and achieve excellent effect of reducing side effects, reducing side effects, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Method of Experiment
[0036]1.1. Preparation of Laboratory Animals
[0037]In this example, as laboratory animals, Sprague-Dawley, Slc: SD rats (6-week-old males, SLC, Shizuoka, Japan) were used. 60 healthy SPF rats were purchased, acclimated for 14 days, and 8 laboratory animals having uniform body weights (intact control: 249.13±12.12 g, 232˜264 g; cisplatin treated group: 225.35±10.58 g, 206˜243 g) when fasting after three weeks of cisplatin administration per each group were selected to be used in the experiment. As shown in Table 2, the laboratory animals were divided into 6 groups. All of the laboratory animals underwent fasting for about 18 hours at each of the first treatment of cisplatin, the first drug administration and the final necropsy (even in this period, drinking water was freely provided), individuals were identified using picric acid.
[0038]To cause a gastrointestinal motility disorder, 2 mg / kg of cisplatin (Sigma-Aldrich, St. Louise, Mo., USA) was disso...
example 2
Confirmation of Changes in Body Weight and Body Weight Gain
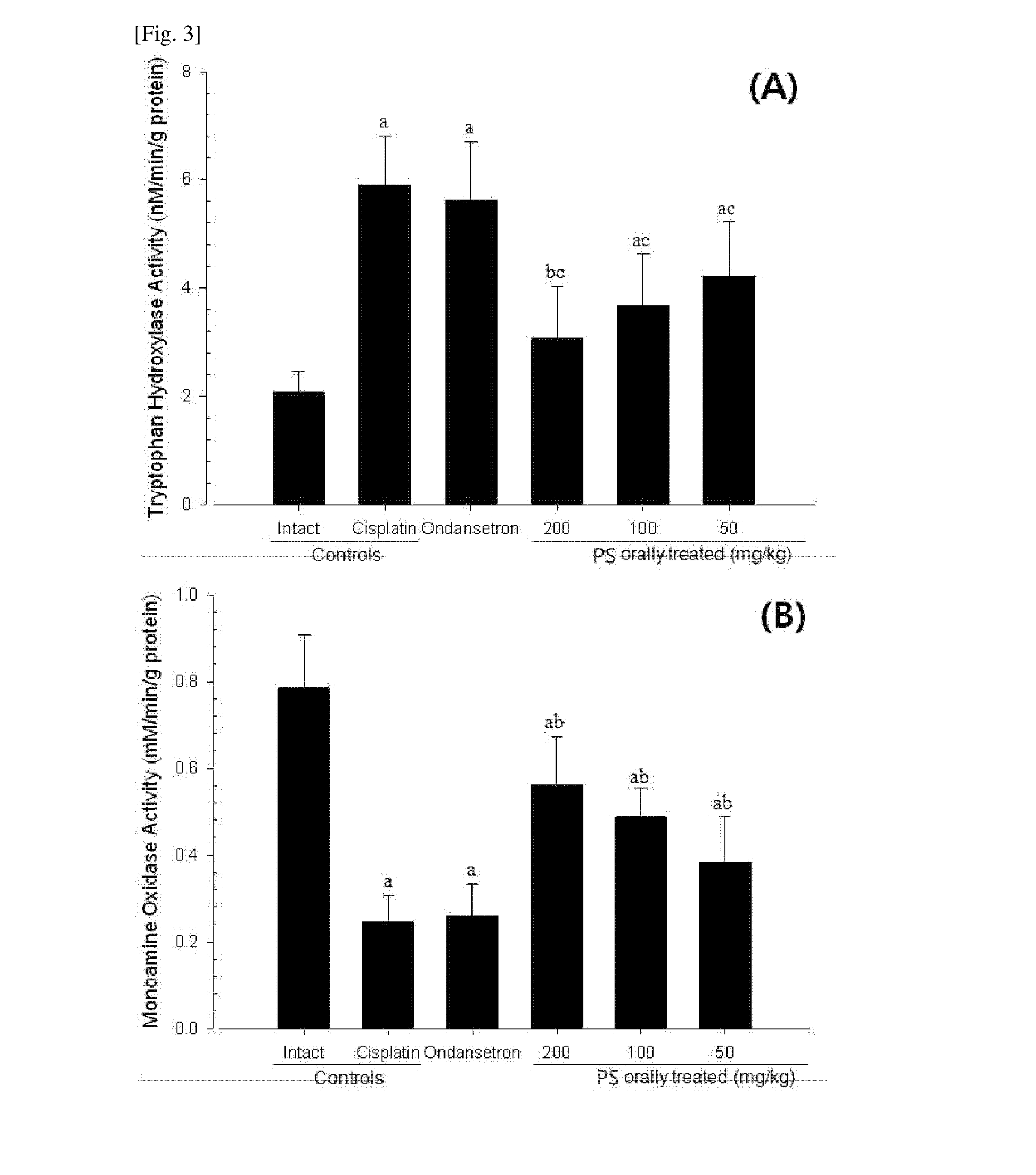
[0042]Body weights and body weight gains in each group that were measured after the first and fourth administrations of cisplatin and the necropsy are shown in Table 3.
TABLE 3Body weight (g)At firstAt 4thBody weight gain (g)cisplatincisplatinTotal experimentTest article dosingGroupstreatment [A]treatment [B]Sacrifice [C]periods [C-A]periods [C-B]ControlsIntact198.38 ± 12.82249.13 ± 12.12 252.88 ± 13.5154.50 ± 5.13 3.75 ± 2.87Cisplatin199.13 ± 14.12225.00 ± 13.90a195.13 ± 12.96a−4.00 ± 7.01 d −29.88 ± 3.04dReferenceOndansetron197.88 ± 11.37226.88 ± 6.60a 212.38 ± 4.66ab14.50 ± 11.64de−14.50 ± 5.68dePS orally administered200 mg / kg199.13 ± 12.02223.63 ± 12.28a231.75 ± 12.89ab32.63 ± 10.27de 8.13 ± 8.22e100 mg / kg199.38 ± 10.46226.38 ± 11.29a218.50 ± 6.89ab19.13 ± 6.62de −7.88 ± 6.40de 50 mg / kg198.25 ± 15.07224.88 ± 10.08a212.00 ± 8.78ac13.75 ± 13.12df −12.88 ± 9.58de
[0043]Three weeks after the first cisplatin administration, ...
example 3
Confirmation of Change in Charcoal Transit Ratio in Small Intestine (Gastrointestinal Motility)
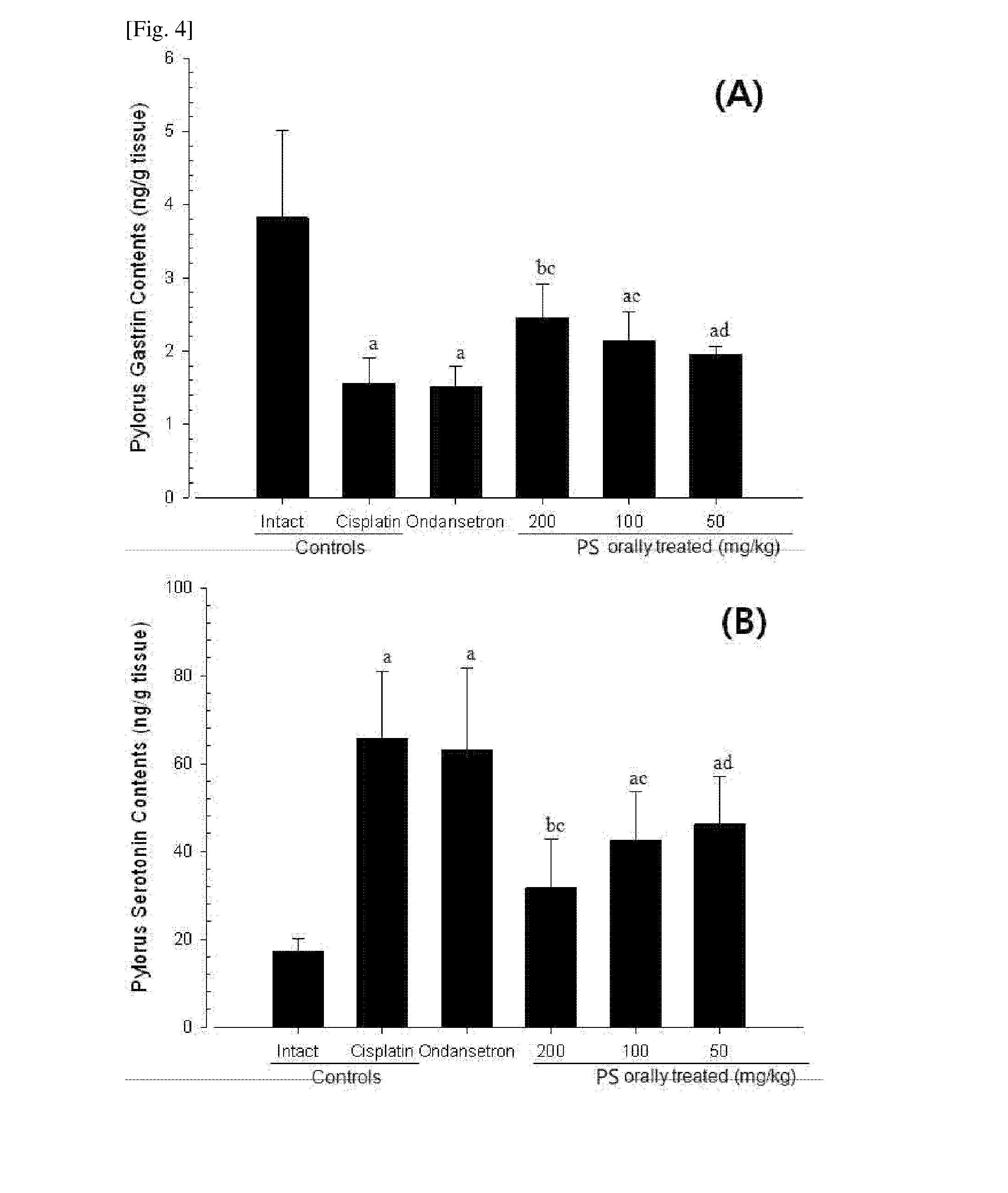
[0047]In a digestive motility disorder, due to reduction in fecal excretion such as constipation and excessive water absorption caused by related fecal retention, it is known that water content in feces is considerably decreased, and fecal parameters such as a fecal pellet number and the water content in the feces have been used to evaluate effects of various drugs on the digestive motility disorder. Also, permeation of a substance through the digestive tract represents the motility in the entire gastrointestinal tract, measurement of a charcoal transit ratio in the small intestine is known to be very useful to diagnose whether or not there is a problem in digestive motility (Wintola et al., 2010), and a decrease in the charcoal transit ratio in the small intestine indicates a decrease in digestive motility such as the fecal retention, that is, constipation (Sagar et al., 2005; Meite et al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com