Fe(III) Complex Compounds For The Treatment And Prophylaxis Of Iron Deficiency Symptoms And Iron Deficiency Anemias
a technology of complex compounds which is applied in the field of complex compounds for the treatment and prophylaxis of iron deficiency symptoms and iron deficiency anemia, can solve the problems of low stability, less suitable for oral application, and nearly not applicable in pharmaceutical compositions, and achieves stable bioavailable iron complexes, treatment and prophylaxis, and low cognitive efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0122]The invention is illustrated in more detail by the following examples. The examples constitute only an exemplary illustration and it lies within the knowledge of a skilled person to extend the specific examples to further claimed compounds. The designation of the example names have been determined with the program ChemDraw Ultra Version 12.0.
Starting Compounds:
[0123]The starting compounds used in the examples were obtained as follows.
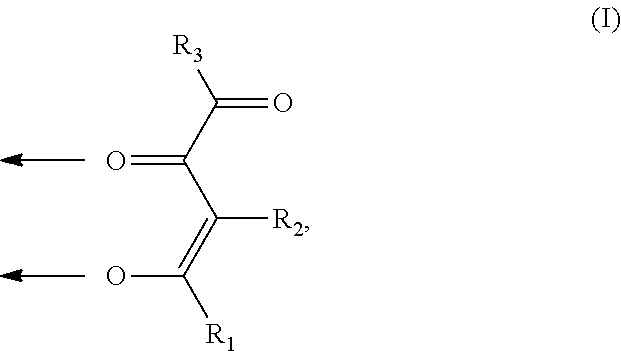
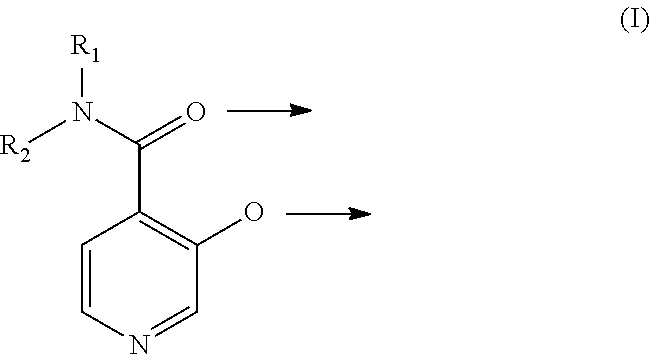
A. 3-Hydroxy-N,N-dimethylisonicotinamide
[0124]
[0125]0.09 mol (15 g) 3-hydroxyisonicotinic acid ethyl ester (in analogy to J. D. Crum, C. H. Fuchsman, J. Heterocyclic Chem. 1966, 3, 252-256) and 0.9 mol dimethylamine (160 ml 5.6 M solution in ethanol) were heated in a pressure vessel to 120° C. for 5 h. Then the reaction mixture was evaporated until dryness and the crude product was recrystallized from 200 ml tetrahydrofuran and 100 ml ethanol. 10 g (67% yield) of the title compound were obtained.
[0126]IR (in substance, cm−1): 1626, 1601, 1573, 150...
preparation examples
Example 1
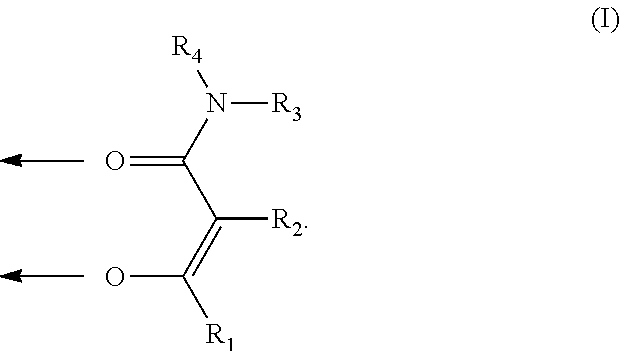
Tris-(3-hydroxy-N,N-dimethylisonicotinamide) iron(III) complex
[0199]
[0200]72 mmol (12 g) 3-hydroxy-N,N-dimethylisonicotinamide were provided in 250 ml ethanol and 72 mmol sodium methanolate (30% solution in methanol) were added. 24 mmol (3.89 g) FeCl3 (anhydrous) were dissolved in 20 ml ethanol, added dropwise and stirred for 2 h. The reaction mixture was filtered off, the filtrate was evaporated at a rotary evaporator and the residue was dried. 13.6 g (96% Fe-yield) of the title compound were obtained.
[0201]IR (in substance, cm−1): 1574, 1525, 1473, 1394, 1308, 1273, 1236, 1206, 1161, 1059, 928, 867, 850, 826, 790, 732, 711, 652, 615.
[0202]CHN-elementary analysis: C, 51.11; H, 5.44; N, 14.37.
[0203]Fe-content: 9.47% [m / m]
2. Tris-(3-Hydroxyisonicotinamide) iron(III) complex
[0204]
[0205]9 mmol (1.37 g) 3-hydroxyisonicotinamide were dissolved in 60 ml methanol under slight heating and 3 mmol (0.49 g) FeCl3 (anhydrous) as well as 9 mmol sodium methanolate (1.67 ml of a 30% solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com